| parameter |
code version |
brief description |
default value |
other comments |
| |
|
|
|
|
 |
ATP_frac |
the amount of ATP used for non-pumping purposes as a fraction of the total ATP being metabolised
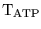 |
5.0000e-01 |
Aubert and Costalat (10) state that in their model, at rest, the pump accounts for about 52 per cent of ATP consumption. p13 of (7) mentions that about fifty percent of normal CMRO2 goes towards homeostasis - i.e. total EEG silence results in about a fifty percent reduction in CMRO2. On p426 of (11) it is estimated that Na-K-ATPase accounts for 70 per cent of brain energy usage. |
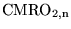 |
CMRO2n |
normal cerebral metabolic rate for oxygen per unit volume of brain water |
3.9456e-02 (fcn) |
a value of about 0.03 can also be calculated using the value of CMRO2 = 3.4 mL/100g brain/min given on this site, or of CMRO2 = 3.4 mL/100g brain/min given here
and using the ideal gas law - i.e. assuming a molar volume for O2 of 24.465 L/mol |
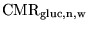 |
CMRglucnw |
normal cerebral metabolic rate for glucose per unit brain weight |
5.2000e-03 |
0.0042 on p415, (12), perhaps we need to assume a little higher in humans to get a slightly higher CMRO2 |
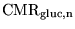 |
CMRglucn |
normal cerebral metabolic rate for glucose per unit volume of brain water |
6.7600e-03 (fcn) |
|
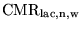 |
CMRlacnw |
normal cerebral metabolic rate for lactate per unit brain weight |
-2.8300e-04 |
-3.8300e-04 on p415, (12) |
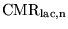 |
CMRlacn |
normal cerebral metabolic rate for lactate per unit volume of brain water |
-3.6790e-04 (fcn) |
We expect this to be small and negative in normal conditions - i.e. small net production of lactate in the brain. |
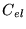 |
C_elec |
A lumped constant equal to RT/F where R is the gas constant, T is body temperature in Kelvin and F is the Faraday constant |
2.6730e+01 |
calculated from values of R, T and F in (13) |
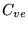 |
C_ve |
compliance of the extracranial venous pathway |
2.3400e+00 |
2.34 in (14) |
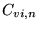 |
C_vin |
normal venous compliance |
4.5930e-01 |
|
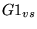 |
G1_vs |
conductance of the terminal intracranial veins when intracranial pressure equals venous sinus pressure |
2.7700e+00 |
2.77
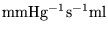 in (14) in (14) |
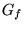 |
G_f |
conductance to CSF formation |
4.2000e-04 |
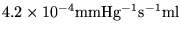 in (14) in (14) |
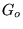 |
G_o |
conductance to CSF outflow |
1.9000e-03 |
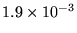 in (14) in (14) |
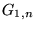 |
G_1n |
normal conductance of the proximal arterial segment |
4.3619e-01 (fcn) |
|
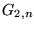 |
G_2n |
normal conductance of the distal arterial segment |
2.6932e-01 (fcn) |
|
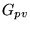 |
G_pv |
conductance of the proximal veins |
1.1360e+00 |
1.136
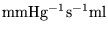 in (14) in (14) |
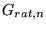 |
G_ratn |
normal ratio of conductance of the proximal to that of the distal arterial segments |
1.6196e+00 |
|
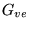 |
G_ve |
conductance of the extracranial venous pathways |
6.2510e+00 |
6.251
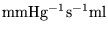 in (14) in (14) |
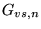 |
G_vsn |
normal conductance of the terminal veins |
1.5740e+00 |
|
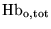 |
Y_tot |
total concentration of amino groups on haemoglobin, able to bind
 . . |
9.0800e+00 (fcn) |
We assume that the interaction of these is independent of the oxygenation of the Hb. It is assumed that there are four such groups per haemoglobin molecule. |
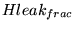 |
Hleak_frac |
the rate at which protons re-enter the mitochondria via leak channels, as a fraction of the total re-entry through leak channels, ATPase and phosphate transport. |
1.0000e-01 |
note that this is the same as a fraction of CMRO2 for isolated mitochondria, but not quite the same for cells, because some protons additionally re-enter while NADH is transported in. Estimates in (15) for this quantity include 20 per cent and 35 per cent. (4) seems to suggest that the proportion is significant in the resting state, but insignificant at maximal ATP producing capacity. |
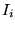 |
I_i |
artificial CSF injection rate |
0.0000e+00 |
a control parameter in the Ursino model. |
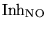 |
Inh_NO |
a parameter reflecting the inibition of NO |
1.0000e+00 |
normal value is 1 |
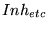 |
Inh_etc |
A parameter which mimics the action of an inhibitor of electron transport such as myxothiazol |
1.0000e+00 |
normal value is 1 |
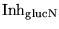 |
Inh_glucN |
the parameter which describes how strongly the AMP/ATP ratio inhibits the conversion of glucose to pyruvate |
3.0000e+00 |
Normal AMP/ATP is about 0.013. Normal activation is 1. Maximum possible activation is (
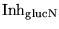 + 1), so for example choosing a value of about 3 means that glycolysis can be upregulated about four times from normal + 1), so for example choosing a value of about 3 means that glycolysis can be upregulated about four times from normal |
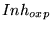 |
Inh_oxp |
a parameter which mimics the actions of an inhibitor of F1-F0-ATPase |
1.0000e+00 |
|
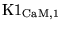 |
K1_CaM1 |
a parameter in the relationship between levels of intracellular calcium and the phosphorylation rate of myosin light chains in the proximal VSM segment |
3.6207e+00 (fcn) |
the maximum rate of myosin light chain phosphorylation (rate limited by the maximum activity of MLCK) |
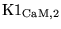 |
K1_CaM2 |
a parameter in the relationship between levels of intracellular calcium and the phosphorylation rate of myosin light chains in the distal VSM segment |
2.7156e+00 (fcn) |
the maximum rate of myosin light chain phosphorylation (rate limited by the maximum activity of MLCK) |
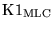 |
K1_MLC |
a parameter in the relationship between force generation and myosin phosphorylation in both VSM segments |
4.0000e+00 (fcn) |
|
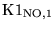 |
K1_NO1 |
a parameter in the relationship between levels of intracellular NO and the dephosphorylation rate of myosin light chains in the proximal VSM segment |
1.1534e+01 (fcn) |
the maximum rate of myosin light chain dephosphorylation (rate limited by the maximum activity of MLCP) |
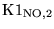 |
K1_NO2 |
a parameter in the relationship between levels of intracellular NO and the dephosphorylation rate of myosin light chains in the distal VSM segment |
1.2255e+01 (fcn) |
the maximum rate of myosin light chain dephosphorylation (rate limited by the maximum activity of MLCP) |
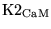 |
K2_CaM |
a parameter in the relationship between levels of intracellular calcium and the phosphorylation rate of myosin light chains in both VSM segments |
3.3000e+00 |
if this parameter is, for example, 5, then this means that the rate is half maximal when intracellular calcium is at five times its normal value. In (16), p359 it is stated that the calcium concentration for half maximal phosphorylation of RLC is 250 nM. This is NOT exactly what we want - we want the calcium conctration for half maximal activation of MLCK, but this is not given. It is however stated that the calcium-calmodulin concentration for the half-maixmal activation of MLCK is 1 nM. Data in (17) suggest that the free Ca/CaM concentration is about 1 nM when calcium concentratin is about 330 nM. If we wish to model the binding of calcium to calmodulin in more detail, this is an important paper to consult. |
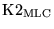 |
K2_MLC |
a parameter in the relationship between force generation and myosin phosphorylation in both VSM segments |
3.0000e+00 |
Make this large and the relationship becomes approximately exponential. Make this small and phosphorylation can make little difference. |
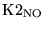 |
K2_NO |
a parameter in the relationship between levels of intracellular NO and the dephosphorylation rate of myosin light chains in both VSM segments |
5.0000e+00 |
if this parameter is, for example, 5, then this means that the rate is half maximal when intracellular NO is at five times its normal value. The value for half maximal activation cGMP is given as about 3.9 nM in (18), which we assume is significantly more than the normal concentration so that increases in NO can have a significant effect. But normal in vivo concentrations in NO which are hard to come by. |
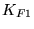 |
K_F1 |
a parameter in the relationship between the rate of oxidative phosphorylation and the membrane and phosphorylation potentials in the Cortassa model |
1.7100e+06 |
We can go with the value in (13) - 1.71e6 - or it can be modified because there, the normal value of phosphate is so much higher than ours... |
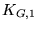 |
K_G1 |
a parameter in the relationship between the conductance and the radius of the proximal cerebral arteries |
1.4300e+06 |
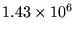 in (14) in (14) |
 |
K_G2 |
a parameter in the relationship between the conductance and the radius of the distal cerebral arteries |
1.0200e+08 |
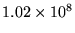 in (14) in (14) |
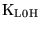 |
K_L0H |
parameter in the expression for the transport of lactate by MCT carriers between extracellular space and blood |
7.5916e-05 (fcn) |
See section 2.3 |
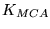 |
K_mca |
a parameter in the relationship between the radius of the middle cerebral artery and the transmural pressure drop |
5.0000e+00 |
5 in (14) |
 |
K_Phosshut |
a parameter in the equation for the transport of phosphate between cytoplasm and mitochondria |
4.7877e-04 (fcn) |
We assume, following Korzeniewski (19), that this carrier is not normally at saturation, and thus set a high value for this parameter. |
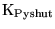 |
K_Pyshut |
a parameter in the equation for the transport of pyruvate between cytoplasm and mitochondria |
1.3725e-05 (fcn) |
|
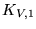 |
K_V1 |
a parameter in the relationship between the volume and the radius of the proximal cerebral arteries |
4.6400e+03 |
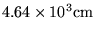 in (14) in (14) |
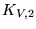 |
K_V2 |
a parameter in the relationship between the volume and the radius of the distal cerebral arteries |
1.5430e+05 |
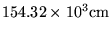 in (14) in (14) |
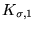 |
K_sigma1 |
a parameter in the relationship between the elastic stress and the inner radius of the proximal cerebral arteries |
1.0000e+01 |
10 in (14) |
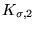 |
K_sigma2 |
a parameter in the relationship between the elastic stress and the inner radius of the distal cerebral arteries |
4.5000e+00 |
4.5 in (14) |
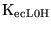 |
K_ecL0H |
parameter in the expression for the transport of lactate by MCT carriers between tissue and extracellular space |
1.1490e-04 (fcn) |
See section 2.3 |
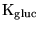 |
K_gluc |
parameter in the expression for the active transport of glucose across the blood-brain barrier |
6.0000e+00 (fcn) |
See section 2.3. See also figure 2.7 (p47) of (3) |
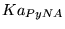 |
Ka_PyNA |
activation constant for ADP for the first steps of the TCA cycle |
6.2000e-02 |
Actually AMP activates pyruvate dehydrogenase and ADP activates isocitrate dehydrogenase, (20) p622. Ka for IDH is given as 6.2e-2 in (13), but the graph in Figure A2 B suggests a considerably lower value |
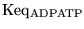 |
k_ADPATP_eq |
equilibrium constant for the conversion of ADP to ATP and AMP |
2.7836e+00 (fcn) |
We are interested in the apparent rather than the true value, as some reactants may not be free. A value of 2.78 for the effective equilibrium constant can be calculated from (5) - see Section 4.1. 0.44 is given in (20), p578, probably for the real constant. The enzyme catalysing the reaction is adenylate kinase. On p816, (20), a contradictory value of 1.2 is quoted. In (21) it is stated that the apparent equilibrium constant depends on magnesium concentrations, but falls in the range of 0.5-1.3 in a variety of tissues. A value of 1.12 for the apparent equilibrium constant is given in Kemp. |
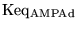 |
k_AMPAd_eq |
equilibrium constant for the degradation of AMP to adenosine |
8.7978e-05 (fcn) |
|
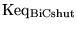 |
k_BiCshut_eq |
equilibrium constant for the transport of bicarbonate ions between mitochondria and cytoplasm |
4.7773e+00 (fcn) |
|
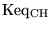 |
k_CH_eq |
equilibrium constant for the production and dissociation of carbonic acid |
7.9000e-04 |
The value quoted in (22) p9 for effective pK of the reaction |
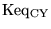 |
k_CY_eq |
equilibrium constant for the combination between
 and the amino groups of haemoglobinunits: and the amino groups of haemoglobinunits:
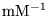 |
4.1118e-01 (fcn) |
|
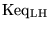 |
k_LH_eq |
equilibrium constant for the dissociation of lactic acid |
1.3800e-01 |
From (20), using the pKa value of 3.86 given on page 46 - changed to our units gives 0.138 mM |
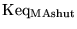 |
Keq_MAshut |
Equilibrium constant for the malate-aspartate shuttle |
1.0000e+01 |
|
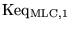 |
k_MLC1_eq |
equilibrium constant for the reaction in which myosin is phosphorylated in the proximal arterial segment |
4.0000e+00 (fcn) |
|
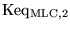 |
k_MLC2_eq |
equilibrium constant for the reaction in which myosin is phosphorylated in the proximal arterial segment |
5.6667e+00 (fcn) |
|
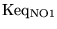 |
k_NO1_eq |
equilibrium contant for the production and loss/degradation of nitric oxide in the proximal arterial segment |
2.0000e-06 (fcn) |
|
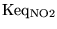 |
k_NO2_eq |
equilibrium contant for the production and loss/degradation of nitric oxide in the distal arterial segment |
2.0000e-06 (fcn) |
|
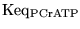 |
k_PCrATP_eq |
equilibrium of the reaction in which phosphocreatine combines with ADP to give creatine and ATP |
6.7137e+04 (fcn) |
calculated assuming that normally this reaction is at equilibrium. The value of 78125
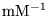 can be calculated from (23). The value in (5) is considerably higher - about can be calculated from (23). The value in (5) is considerably higher - about
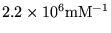 . So is the value in (10) which translates to about . So is the value in (10) which translates to about
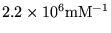 (including protons in the equation) - but this doesn't seem to square with the estimates of the concentrations of the metabolites and the fact that this reaction should be at equilibrium. It implies that (including protons in the equation) - but this doesn't seem to square with the estimates of the concentrations of the metabolites and the fact that this reaction should be at equilibrium. It implies that
![$\ensuremath{\mathrm{[ATP][Cr]/([ADP][PCr])}}$](img408.png) is about 200, suggesting much lower values of ADP than we assume from the other references. is about 200, suggesting much lower values of ADP than we assume from the other references. |
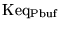 |
k_Pbuf_eq |
Equilibrium constant for the reaction in which cellular proteins bind protons |
4.2557e-04 (fcn) |
|
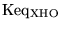 |
k_XHO_eq |
equilibrium constant for the combination of protonated haemoglobin with oxygen |
8.7115e+02 (fcn) |
See section 4.3. The ratio of this value to
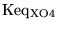 must be the same as that of must be the same as that of
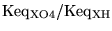 - see the theory in Section 16.2.2 of (3) - see the theory in Section 16.2.2 of (3) |
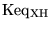 |
k_XH_eq |
equilibrium constant for the combination of haemoglobin with protons |
7.6923e+04 (fcn) |
See section 4.3. The value is calculated from the pK value of 7.9 given on this site |
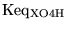 |
k_XO4H_eq |
equilibrium constant for the combination of oxyhaemoglobin with protons |
5.0000e+03 (fcn) |
See section 4.3. The value is calculated from the pK value of 6.7 given on this site |
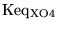 |
k_XO4_eq |
equilibrium constant for the combination of haemoglobin with oxygen |
1.3402e+04 (fcn) |
See section 4.3 |
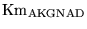 |
Km_AKGNAD |
Km for ADP for the (caricatured) second stage of the TCA cycle in which
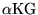 is converted to succinate is converted to succinate |
8.2677e-02 (fcn) |
(13), like ourselves, assume rapid equilibriation between the ATP and GTP pools, making it valid to consider ADP a substrate for the succinyl-CoA synthase reaction. However they do not give ADP or succinyl-CoA Km values, and use instead mass action kinetics. Because the succinate lyase reaction is considered normally to be at equilibrium, we can assume that it is not rate limiting, and that until we get to very low concs of ADP, it does not affect the reaction. |
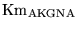 |
Km_AKGNA |
Km for
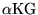 for the (caricatured) second stage of the TCA cycle in which for the (caricatured) second stage of the TCA cycle in which
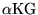 is converted to succinate is converted to succinate |
3.0000e-01 (fcn) |
In (13), a Km for alpha-ketoglutarate of 1.94 mM is given for the alpha-ketoglutarate dehydrogenase reaction. There is also a hill coefficient (1.2) involved. But the graph presented in A3 A strongly contradicts the value given, and suggests a value more like 1.94 micromoles (i.e. normally at saturation - perhaps a misprint?). However if we have a value which is considerably lower than the normal cellular concentration this makes the equilibrium concentration of the metabolite very sensitive to changes in the overall rate. There is also no other way of upregulating the TCA cycle if this reaction is running close to maximum for levels of AKG and NAD. Unless we introduce aspartate amino transferase as in cortassa... |
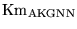 |
Km_AKGNN |
Km for NAD for the (caricatured) second stage of the TCA cycle in which
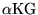 is converted to succinate is converted to succinate |
2.7273e+01 (fcn) |
In (13) a Km for NAD of 38.7 mM is given for the alpha-ketoglutarate dehydrrogenase reaction. (Note the very poor notation.) This means that the reaction is pretty much linearly dependent on NAD at all possible mitochondrial concentrations |
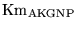 |
Km_AKGNP |
Km for phosphate for the (caricatured) second stage of the TCA cycle in which
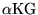 is converted to succinate is converted to succinate |
6.4000e-01 (fcn) |
See argument for Km for ADP for this reaction. In (13), inorganic phosphate appears never to be considered rate limiting for anything. This could essentially imply either that the pool is kept reasonably constant, or that there are low Km values. The model suggests that there can be significant variation in the mitochondrial levels of phosphate, suggesting the latter reason. Moreover the model is quite sensitive to this parameter and can crash if drops in mitochondrial phosphate levels (caused by depolarisation of the membrane and hence slower transport) have too dramatic an effect on the TCA cycle. |
 |
Km_ATPuse_frac |
fraction of normal level of ATP at which we have half maximal use of ATP in the reaction where ATP is used for non-pumping processes |
1.0000e-01 |
|
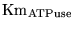 |
Km_ATPuse |
level of ATP at which we have half maximal use of ATP in the reaction where ATP is used for non-pumping processes |
3.0000e-01 (fcn) |
Korzeniewski (5) uses a value of 0.15 mM, though this is for muscle and is for all ATP use. If this parameter is low compared to normal levels of ATP, then we are operating near saturation, and a drop in ATP will cause little change in the rate of use. This could mean however that there is a greater effect on the pumping of potassium, and extracellular potassium rises. |
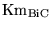 |
Km_BiC |
Km for the transport of bicarbonate between blood and extracellular space |
2.5060e+01 (fcn) |
|
 |
Km_Caout_ratio |
ratio of
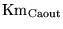 to the normal concentration of Calcium in a muscle cell to the normal concentration of Calcium in a muscle cell |
1.0000e+01 |
A large value of
 implies that calcium extrusion from smooth muscle cells doesn't saturate quickly implies that calcium extrusion from smooth muscle cells doesn't saturate quickly |
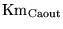 |
Km_Caout |
Km value for the extrusion of calcium from muscle cells |
1.0000e-03 (fcn) |
In the two-pool model in chapter 5 of (3) calcium extrusion has two mechanisms. Extrusion from the cell is not given any saturation. Sequestration in internal stores is given saturable dynamics with a Km of about 0.001 mM (i.e. about ten times the normal calcium concentration). |
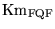 |
Km_FQF |
Km for the
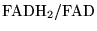 ratio in the oxidation of ratio in the oxidation of
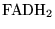 |
2.4400e+01 (fcn) |
In (13)
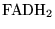 is treated as a parameter because the sensitivity of overall oxygen consumption to complex II is very low, suggesting perhaps that this has a low Km compared to normal values is treated as a parameter because the sensitivity of overall oxygen consumption to complex II is very low, suggesting perhaps that this has a low Km compared to normal values |
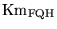 |
Km_FQH |
Km for the ratio of proton concentrations in the oxidation of
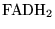 |
2.5119e-01 (fcn) |
|
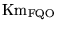 |
Km_FQO |
Km for oxygen in the oxidation of
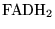 |
1.1375e-03 (fcn) |
in (13) it is stated that "the sensitivity of the overall oxygen consumption with complex II is very low" - this could mean that this quantity is small. |
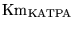 |
Km_KATPA |
Km for intracellular ATP for the sodium potassium pump |
1.4000e+00 |
From (24) Na,K-ATPase has two binding sites for ATP a high affinity site with Km about 0.004 mM and a low affinity site with Km about 1.4 mM which display negative cooperativity. The first of these is presumably always saturated. From the graph in Fig. 1. the first of the low affinity site appears to dominate. 0.23 mM given for cortical cell cultures from rats in (25). 0.5 mM used in (10). |
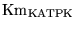 |
Km_KATPK |
Km for extracellular potassium for the sodium potassium pump |
1.0889e-02 (fcn) |
Km values of between about 1 and about 2.8 are given in (26). Note that this Km is for the ratio of extracellular to intracellular potassium. |
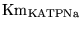 |
Km_KATPNa |
Km for intracellular sodium for the sodium potassium pump |
2.1739e-01 (fcn) |
Km values of between about 8 and about 30 are given in (26). Note that our Km is for the ratio of intracellular to extracellular sodium. |
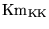 |
Km_KK |
Km for the transport of potassium ions between blood and extracellular space |
2.8000e+00 (fcn) |
|
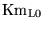 |
Km_L0 |
Km for the MCT lactate transporter at the BBB |
1.8000e+00 |
1.8 +- 0.6 mM - p121 (11) |
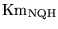 |
Km_NQH |
Km for the ratio of proton concentrations in the oxidation of NADH |
2.5119e-01 (fcn) |
This parameter actually represents the way that the rate of electron transport depends on the proton motive force. If we assume that the membrane potential is proportional to the pH gradient, then the Km formalism should be okay. |
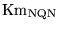 |
Km_NQN |
Km for the NADH/NAD ratio in the oxidation of NADH |
2.0000e-01 (fcn) |
In (13) a complicated relationship is presented. However figure A5 A of this reference suggests that the rate of oxygen use saturates with incresing NADH, suggesting that it is reasonable to model using the Km formalism. Half maximal NADH estimated from this graph is perhaps about 0.05 mM. This is however one of the pathways by which the TCA cycle can control the rate of oxidative phosphorylation. A low Km value compared to normal values suggests that upregulating the TCA cycle can't really upregulate the electron transport chain. |
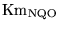 |
Km_NQO |
Km for oxygen in the oxidation of NADH |
5.0000e-02 |
This parameter controls when the cell starts to "feel" hypoxia. There seems to be some disagreement about the way that the rate of oxidative phosphorylation depends on the oxygen tension in mitochondria. (27) gives a value of 0.8 mmHg for human neuroblastoma cells which translates to 0.0011 mM if oxygen solubility is about 0.0014 mM/mmHg. Chapter 5 of (7) suggests slightly lower values. |
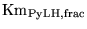 |
Km_PyLH_frac |
Km for protons in the conversion of pyruvate to lactate as a proportion of normal hydrogen ion concentration |
2.0000e+00 |
|
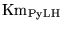 |
Km_PyLH |
Km for protons in the conversion of pyruvate to lactate |
1.7023e-04 (fcn) |
if this parameter is reasonably high then that allows the more rapid conversion of pyruvate to lactate during ischaemia. |
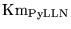 |
Km_PyLLN |
Constant representing the interaction of lactate and NAD in the rate of conversion of pyruvate to lactate |
2.4000e-01 |
0.24
 from (28) from (28) |
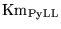 |
Km_PyLL |
Km for lactate in the conversion of lactate to pyruvate |
2.0000e+00 |
2 mM in (28). Values of 8.62 - 21.9 mM are given for mice in (29). |
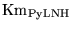 |
Km_PyLNH |
Km for NADH in the conversion of pyruvate to lactate |
6.2000e-03 |
0.0062 mM given in (28) |
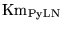 |
Km_PyLN |
Km for NAD in the conversion of lactate to pyruvate |
3.0000e-02 |
0.03 mM in (28) |
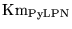 |
Km_PyLPN |
Constant representing the interaction of pyruvate and NADH in the rate of conversion of pyruvate to lactate |
3.4000e-05 |
3.4e-5
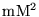 from (28) from (28) |
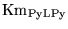 |
Km_PyLPy |
Km for pyruvate in the conversion of pyruvate to lactate |
3.4000e-02 |
values of 0.03 and 0.39 mM are given in BRENDA for homo sapiens. 0.034 given in (28) |
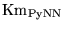 |
Km_PyNN |
Km for NAD for the (caricatured) first stage of the TCA cycle, in which pyruvate and oxaloacetate are converted to
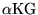 |
9.0909e-01 (fcn) |
In (30) a Km of 0.7 for NAD is given for PDH for a mouse tumour cell line. In (13), a Km for NAD of 0.923 is given for the isocitrate dehydrogenase reaction (note the very poor notation!) |
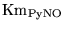 |
Km_PyNO |
Km for oxaloacetate for the (caricatured) first stage of the TCA cycle, in which pyruvate and oxaloacetate are converted to
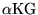 units: mM units: mM |
6.4000e-04 |
in (13), Table 3 the value of 6.4e-4 is given for the Km for oxaloacetate of citrate synthase. However if we have a value which is considerably lower than the normal cellular concentration this makes the equilibrium concentration of the metabolite very sensitive to changes in the overall rate. |
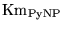 |
Km_PyNP |
Km for pyruvate for the (caricatured) first stage of the TCA cycle, in which pyruvate and oxaloacetate are converted to
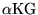 |
3.8218e-02 (fcn) |
The reaction is catalysed by pyruvate dehydrogenase (PDH) On p146 of (11), a Km value of 0.05 is given. In (30), Km values of 0.017-0.036 mM are given for a mouse tumour cell-line depending on the pH. 0.025 mM is given in this abstract
However PDH is activated by AMP (Ka = 0.04 mM), inhibited by its product AcCoA, (Ki = 0.06 mM) and inhibited by NADH with a Ki of 0.08 mM. Thus increased demand (via increased AMP) should activate it... |
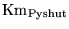 |
Km_Pyshut |
Km for the transport of pyruvate into mitochondria |
2.1500e-01 |
The only value we have is 0.215 mM for the transport of pyruvate into mitochondria in young rat hearts in Paradies et al (31) |
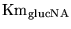 |
Km_glucNA |
Km for ADP in the caricature of glycolysis |
7.0000e-02 (fcn) |
ADP is used as a substrate in two reactions - the first is the conversion of 1-3, biphosphoglycerate to 3-P-glycerate by phosphoglycerate kinase, and the second is the conversion of phosphoenolpyruvate to pyruvate by pyruvate kinase. In (32), ADP doesn't come into the rate equation for the first of these, and for the second of these comes in in a very complicated way. |
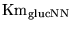 |
Km_glucNN |
Km for NAD in the caricature of glycolysis |
1.0000e+00 (fcn) |
(32) (held as glycomodel.pdf) gives a value of 0.18 for the Km for NAD in the conversion of glyceraldehyde-3-P to 1,3-biphosphoglycerate by the enzyme Glyceraldehyde-3-P dehydrogenase (1.2.1.59) |
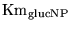 |
Km_glucNP |
Km for inorganic phosphate in the caricature of glycolysis |
3.0000e-01 (fcn) |
|
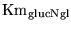 |
Km_glucNgl |
Km for glucose in the caricature of glycolysis |
5.0000e-02 |
Km of Hexokinase for glucose is 0.05 mM on p 143 of (11); On p364 of (20), a Km 0.15 mM is given for Hexokinase for glucose |
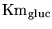 |
Km_gluc |
Km for the active transport of glucose across the blood-brain barrier |
8.0000e+00 |
8.0 mM on p 115, (12); 11.0 +- 1.4 mM on p121 of (11) |
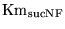 |
Km_sucNF |
Km for FAD for the (caricatured) third stage of the TCA cycle in which succinate is converted to oxaloacetate |
1.0000e-01 (fcn) |
In (13), no information on how the concentration of FAD affects the succinate dehydrogenase reaction is given. But if the reaction is overly sensitive to the concentration of FAD, which seems to be anyway low, then any decrease in FAD can rapidly bring the TCA cycle to a halt. On the other hand, this is one of the ways that the electron transport chain can have a feedback effect on the TA cycle... |
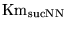 |
Km_sucNN |
Km for NAD for the (caricatured) third stage of the TCA cycle in which succinate is converted to oxaloacetate |
4.5455e-01 (fcn) |
This really represents the Km for NAD for Malate Dehydrogenase which is given in (13) as 0.2244 mM |
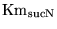 |
Km_sucNs |
Km for succinate for the (caricatured) third stage of the TCA cycle in which succinate is converted to oxaloacetate |
3.0000e-01 (fcn) |
in (13), a Km for succinate of 3.0e-2 is given for the succinate dehydrogenase reaction. However the reaction is also inhibited by the product (fumarate). Data at sun.science.wayne.edu/ bio669/bio6160/6160ps2.pdf suggests a Km of about 0.2 |
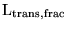 |
L_trans_frac |
lactate which is transported in undissociated form as a fraction of total lactate outflow |
5.0000e-02 |
this parameter has a significant stabilising effect during ischaemia, allowing efflux of acid and prevent excessive acidification of the cell |
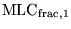 |
MLC1_inac_fracn |
fraction of myosin light chains which are unphosphorylated in normal circumstances in the proximal VSM segment |
8.0000e-01 |
|
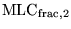 |
MLC2_inac_fracn |
fraction of myosin light chains which are unphosphorylated in normal circumstances in the distal VSM segment |
8.5000e-01 |
|
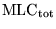 |
MLC_tot |
total concentration of myosin in VSM cells |
5.6000e-02 |
0.056 mM is given on p64 of Barany |
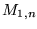 |
M_1n |
normal level of muscle activation in proximal arterial segment |
1.0000e+00 |
this is normally 1 by definition |
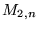 |
M_2n |
normal level of muscle activation in distal arterial segment |
1.0000e+00 |
this is normally 1 by definition |
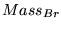 |
Mass_Br |
brain mass |
1.3000e+03 |
1300-1400 g at http://faculty.washington.edu/chudler/facts.html. Note that we are only really interested in the density being correct - the mass is never used outside this context. |
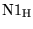 |
N1_H |
the number of protons pumped out of the mitochondrial matrix during the oxidation of two molecules of NADH |
2.0000e+01 |
See section 3 |
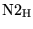 |
N2_H |
the number of protons pumped out of the mitochondrial matrix during the oxidation of two molecules of
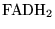 |
1.6000e+01 |
See section 3 |
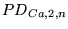 |
PD_Ca2n |
normal membrane potential for calcium ions in the distal VSM segment |
1.5387e+02 (fcn) |
See section 5.4. 150 mV in (33) (Fig. 1) |
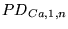 |
PD_Ca1n |
normal membrane potential for calcium ions in the proximal VSM segment |
1.5387e+02 (fcn) |
See section 5.4. 150 mV in (33) (Fig. 1) |
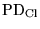 |
PD_Cl |
membrane potential for VSM cells for chloride ions. |
-2.0000e+01 |
-20.0 mV in (11) p74 for cerebral VSM; -30.0 mV in (8), p47 for frog muscle; -31 mV in (33) (Fig. 1) |
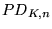 |
PD_Kn |
normal membrane potential for potassium ions in both segments |
-1.1129e+02 (fcn) |
-84 mV in (33) |
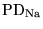 |
PD_Na |
membrane potential for VSM cells for sodium ions. |
4.0000e+01 |
62.6 in (8) p47 frog muscle; 58 mV in (33) (Fig. 1) |
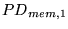 |
PD_mem1n |
normal membrane potential at physiological transmural pressure for the proximal VSM segment |
-5.0000e+01 |
Chrissobolis et al. (34) give a resting membrane potential of about -57 for the rat. (11) chapter 4, p72 suggest a resting membrane potential of -45 to -35 mV. |
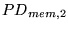 |
PD_mem2n |
normal membrane potential at physiological transmural pressure for the distal VSM segment |
-5.3000e+01 |
see comments for PD_mem1 |
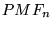 |
PMFn |
normal value of the proton motive force |
1.8693e+02 (fcn) |
|
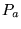 |
P_a |
arterial blood pressure |
1.0000e+02 |
|
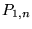 |
P_1n |
normal blood pressure in the proximal arterial segment. |
8.5671e+01 (fcn) |
Just taken from model in normal state! |
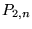 |
P_2n |
normal blood pressure in the distal arterial segment. |
4.8136e+01 (fcn) |
just taken from model in normal state |
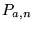 |
P_an |
normal arterial blood pressure |
1.0000e+02 |
|
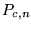 |
P_cn |
normal blood pressure in the capillary segment. |
2.4929e+01 (fcn) |
Just taken from model in normal state |
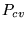 |
P_cv |
central venous pressure |
4.0000e+00 |
4 mmHg in (14) |
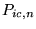 |
P_icn |
normal intracranial pressure |
9.4265e+00 (fcn) |
|
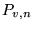 |
P_vn |
normal venous pressure |
1.3937e+01 (fcn) |
|
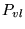 |
P_vl |
transmural pressure value (usually slightly negative) at which cerebral veins collapse |
-2.5000e+00 |
-2.5 mmHg in (14) |
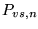 |
P_vsn |
normal venous sinus pressure |
5.9997e+00 (fcn) |
|
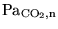 |
Pa_CO2n |
normal arterial partial pressure of
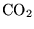 |
4.0000e+01 |
baseline values of about 40 mmHg are very common - see for example chapter 24 of (11). 35 - 45 mmHg at this site |
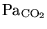 |
Pa_CO2 |
arterial partial pressure of
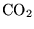 |
4.0000e+01 |
a control parameter |
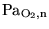 |
PaO2n |
normal arterial partial pressure of oxygen |
9.5000e+01 |
p 415 (12) - 89.4, table 61. Ilias, microdialysis results: 90 - 110 mmHg. 75 - 100 mmHg at this site |
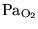 |
PaO2 |
arterial partial pressure of oxygen |
9.5000e+01 |
a control variable |
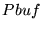 |
Pbufn |
Normal cytoplasmic concentration of sites on cellular proteins capable of binding protons |
1.0000e+01 |
a guess |
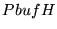 |
PbufHn |
Normal concentration of sites on cellular proteins bound to protons |
2.0000e+00 |
a guess |
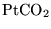 |
PtCO2n |
partial pressure of
 in tissue in tissue |
5.4000e+01 |
46.0 mmHg in (12), p315, in (2) 50.5 mmHg in CSF (i.e. we expect it to be higher in tissue) |
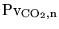 |
PvCO2n |
normal venous partial pressure of
 |
5.1000e+01 |
see (12), p315 |
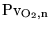 |
PvO2n |
normal venous partial pressure of
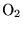 |
3.5800e+01 |
see (12) p232 (and p489), |
 |
nadnadh1_ratio |
the normal cytoplasmic ratio of NAD to NADH |
1.0000e+02 |
A ratio of about 9.14 can be calculated from (7), p 45 for mice brains. However since the majority of NAD is contained in the mitochondria, this doesn't tell us a lot about the cytoplasmic state. [On this website
30-50 is given for yeast.] |
 |
nadnadh2_ratio |
the normal mitochondrial ratio of NAD to NADH |
1.0000e+01 |
a value of 20 is given on (20) |
 |
Rbf_BiC |
ratio of backward to forward rates of transport of bicarbonate ions from blood to extracellular space |
8.0000e-01 |
a guess |
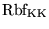 |
Rbf_KK |
ratio of forward to backward rates of transport of potassium ions from blood to extracellular space |
9.0000e-01 |
this parameter essentially reflects the amount of potassium transporter. If it is close to one then potassium rapidly equilibriates across the BBB. Otherwise during hypoxia potassium can build up in the ecs to levels where it starts to act as a vasoconstrictor and we get a sudden "death" transition. |
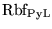 |
Rbf_PyL |
ratio of backward to forward rates in the conversion of lactate to pyruvate |
9.5000e-01 |
See section 2.1. The value of this parameter is worth exploring, as the model behaviour during ischaemia (particularly the lactate to pyruate ratio) is sensitve to it as it approaches 1. |
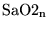 |
SaO2n |
normal arterial oxygen saturation |
9.6278e-01 (fcn) |
|
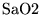 |
SaO2 |
arterial oxygen saturation |
9.6278e-01 (fcn) |
|
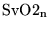 |
SvO2n |
normal venous oxygen saturation |
6.1515e-01 (fcn) |
calculated using data on normal CMRO2 and normal blood flow |
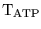 |
netATPuse |
total usage of ATP by the brain in normal circumstances per unit volume of brain water |
1.9502e-01 (fcn) |
See section 3. This depends both on the production of ATP directly from glycolysis and the TCA cycle, and on the oxidation of NADH and FADH2. |
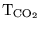 |
netCO2prodn |
net production of CO2 under normal conditions per unit volume of brain water |
3.9456e-02 (fcn) |
See section 3. |
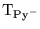 |
netPyusen |
net metabolic rate of pyruvate under normal conditions per unit volume of brain water |
1.3152e-02 (fcn) |
See section 3. |
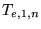 |
T_e1n |
normal elastic tension in the vessel walls of the proximal arterial segment |
-4.3856e-02 (fcn) |
|
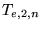 |
T_e2n |
normal elastic tension in the vessel walls of the distal arterial segment |
-1.1205e-01 (fcn) |
|
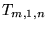 |
T_m1n |
normal active tension in the vessel walls of the proximal arterial segment |
1.8087e+00 (fcn) |
|
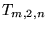 |
T_m2n |
normal active tension in the vessel walls of the distal arterial segment |
3.6079e-01 (fcn) |
|
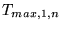 |
T_max1n |
maximum active muscle tension in the proximal arterial segment under basal conditions |
2.1600e+00 (fcn) |
|
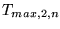 |
T_max2n |
maximum active muscle tension in the distal arterial segment under basal conditions |
1.5000e+00 (fcn) |
|
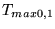 |
T_max01 |
normal maximum smooth muscle tension in the proximal arterial segment |
2.1600e+00 |
|
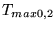 |
T_max02 |
normal maximum smooth muscle tension in the distal arterial segment |
1.5000e+00 |
|
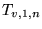 |
T_v1n |
normal viscous tension in the vessel walls of the proximal arterial segment |
0.0000e+00 |
|
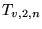 |
T_v2n |
normal viscous tension in the vessel walls of the distal arterial segment |
0.0000e+00 |
|
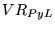 |
VR_PyL |
ratio of the backward to forward Vmax values in the conversion of pyruvate to lactate |
4.3537e-01 (fcn) |
One can calculate a value of about 4 from (28). But at these substrate concentrations that would imply that the normal direction of the reaction is from lactate to pyruvate. So we use a smaller value. |
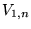 |
V_1n |
normal blood volume in the proximal arterial segment |
2.5626e+00 (fcn) |
|
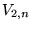 |
V_2n |
normal blood volume in the distal arterial segment |
7.9286e+00 (fcn) |
|
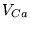 |
V_Ca |
a parameter in the relationship between membrane potential and the probability of a calcium channel being open |
-2.3500e+01 |
Taken from (35) (there called 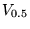 ) ) |
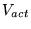 |
V_act |
a parameter in the relationship between membrane potential and the probability of a voltage-gated potassium channel being open |
-9.0000e+00 |
-9 mV in (33); -3.5 mV given for rat VSM in (36), |
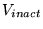 |
V_inact |
a parameter in the relationship between membrane potential and the probability of a voltage-gated potassium channel being open |
-3.5000e+01 |
range from -25 to -45 mV given in (33); -33.9 mV given for rat VSM in (36) |
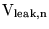 |
V_leakn |
The rate of proton leak through the mitochondrial membrane for whole cells respiring at normal rate (per unit volume of brain water) |
7.6282e-02 (fcn) |
See section 3. |
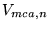 |
V_mcan |
normal approximate velocity of the blood in the middle cerebral artery |
5.8946e+01 (fcn) |
|
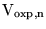 |
V_oxpn |
The normal rate of oxidative phosphorylation in complete cells (per unit volume of brain water) |
1.6835e-01 (fcn) |
See section 3. |
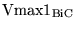 |
Vmax1_BiC |
Vmax for the transport of bicarbonate from blood to extracellular space |
1.6095e+00 (fcn) |
methodology of Section 2.1 |
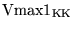 |
Vmax1_KK |
Vmax for the transport of potassium ions from blood to extracellular space |
3.0992e-01 (fcn) |
methodology of Section 2.1 |
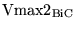 |
Vmax2_BiC |
Vmax for the transport of bicarbonate from extracellular space to blood |
1.3053e+00 (fcn) |
methodology of Section 2.1 |
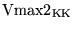 |
Vmax2_KK |
Vmax for the transport of potassium ions from extracellular space to blood |
3.2815e-01 (fcn) |
methodology of Section 2.1 |
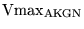 |
Vmax_AKGN |
Vmax for the (caricatured) second stage of the TCA cycle in which
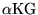 is converted to succinate is converted to succinate |
3.4722e+00 (fcn) |
|
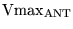 |
Vmax_ANT |
Vmax for the adenine nucleotide transporter |
4.9378e+02 (fcn) |
we require
![$\ensuremath{\mathrm{\frac{[ATP_{im}]}{[ADP_{im}]}}}$](img523.png) 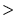
![$\ensuremath{\mathrm{k1_{ANT}\frac{[ATP_{cyt}]}{[ADP_{cyt}]}}}$](img525.png) for the transport to proceed in the correct direction at normal concentrations. This wouldn't be the case if as stated in (12) the mitochondrial ratio was significantly less than the cytoplasmic, but (37) suggests that this result is wrong in any case. for the transport to proceed in the correct direction at normal concentrations. This wouldn't be the case if as stated in (12) the mitochondrial ratio was significantly less than the cytoplasmic, but (37) suggests that this result is wrong in any case. |
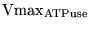 |
Vmax_ATPuse |
Vmax for the reaction in which ATP is used for non-pumping purposes |
1.4897e-01 (fcn) |
See Section 3.1. |
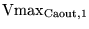 |
Vmax_Caout1 |
Vmax for the extrusion of calcium from the cytoplasm of the proximal VSM segment |
1.5249e-04 (fcn) |
see section 4.4 |
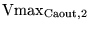 |
Vmax_Caout2 |
Vmax for the extrusion of calcium from the cytoplasm of the distal VSM segment |
1.5249e-04 (fcn) |
see section 4.4 |
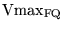 |
Vmax_FQ |
Vmax for the oxidation of
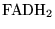 |
4.1429e+01 (fcn) |
methodology of section 2.1 |
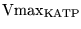 |
Vmax_KATP |
Vmax for the sodium potassium pump |
7.2937e+02 (fcn) |
See Section 3.1. (26) is very detailed, but does not discuss the ATP sensitivity of the ATPases |
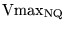 |
Vmax_NQ |
Vmax for the oxidation of NADH |
6.3087e+02 (fcn) |
methodology of section 2.1 |
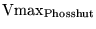 |
Vmax_Phosshut |
a parameter in the equation for the transport of phosphate between cytoplasm and mitochondria |
1.8656e+00 (fcn) |
methodology of section 2.1 |
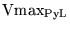 |
Vmax_PyL |
Vmax in the forward direction for the conversion of pyruvate to lactate |
5.5860e-02 (fcn) |
Methodology of section 2.1 |
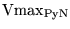 |
Vmax_PyN |
Vmax for the (caricatured) first stage of the TCA cycle, in which pyruvate and oxaloacetate are converted to
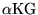 |
1.9658e-01 (fcn) |
|
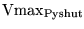 |
Vmax_Pyshut |
a parameter in the equation for the transport of pyruvate between cytoplasm and mitochondria |
5.3997e-03 (fcn) |
methodology of section 2.1 |
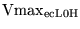 |
Vmax_ecL0H |
parameter in the expression for the transport of lactate by MCT carriers between intracellular to extracellular spaces |
5.1035e-04 (fcn) |
|
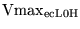 |
Vmax_L0H |
parameter in the expression for the transport of lactate by MCT carriers between blood and extracellular space |
1.8323e-04 (fcn) |
|
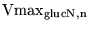 |
Vmax_glucNn |
normal Vmax for glycolysis which is assumed to be modified by the ratio of AMP to ATP |
1.2973e-01 (fcn) |
methodology of section 2.1. |
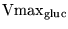 |
Vmax_gluc |
parameter in the expression for the active transport of glucose across the blood-brain barrier |
3.5487e+02 (fcn) |
methodology of section 2.1 |
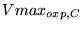 |
VmaxC_oxp |
a parameter in the relationship between the rate of oxidative phosphorylation and the membrane and phosphorylation potentials in the Cortassa model |
-3.4982e+01 (fcn) |
methodology of section 2.1 |
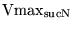 |
Vmax_sucN |
Vmax for the (caricatured) third stage of the TCA cycle in which succinate is converted to oxaloacetate |
1.3810e+00 (fcn) |
|
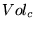 |
Vol_c |
volume of the cerebral capillaries |
6.1750e+00 (fcn) |
|
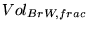 |
Vol_BrW_frac |
brain water as a fraction of total brain volume |
8.0000e-01 |
Seems to be standard. 0.77-0.78 at this site. Or can be derived from (38), p30 where the value in ml/g is given |
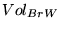 |
Vol_BrW |
total volume of brain water |
1.0000e+03 (fcn) |
|
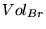 |
Vol_Br |
total brain volume |
1.2500e+03 |
in ml from this site |
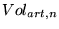 |
Vol_artn |
normal volume of arterial blood in tissue |
1.0491e+01 (fcn) |
must be compatible with Ursino model. (39) give a value of 25 ml in table 1 with a value of 50 ml for 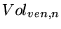 . For our parameter values this assumes a total blood volume of 6 per cent - somewhat higher than our estimates . For our parameter values this assumes a total blood volume of 6 per cent - somewhat higher than our estimates |
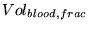 |
Vol_blood_frac |
fraction of normal brain water volume that is blood |
4.7500e-02 |
If 4 per cent of the brain is blood, this translates to 5 per cent of the brain water. In (11) it is stated on p 40 that 6 per cent of the brain volume is blood. On p15 of (7) 2-5 per cent is given. |
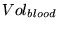 |
Vol_blood |
normal volume of blood in the brain |
4.7500e+01 (fcn) |
|
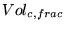 |
Vol_c_frac |
volume of the capillaries as a fraction of the total total blood in the brain |
1.3000e-01 |
1 per cent of brain volume is quoted on p232 of (12). |
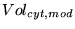 |
Vol_exm_mod |
a control parameter |
1.0000e+00 |
|
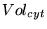 |
Vol_exm |
volume of the extra-mitochondrial space (cell cytoplasm) |
7.2000e+02 (fcn) |
|
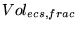 |
Vol_ecs_frac |
extracellular space as a fraction of 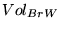 |
2.0000e-01 |
given as 0.1-0.15 on p15 of (7) |
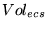 |
Vol_ecs |
volume of the extracellular space |
2.0000e+02 (fcn) |
CSF volume is given as about 125-150 ml on this site, so a value of about 150-200 ml seems reasonable for this parameter |
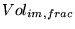 |
Vol_inm_frac |
intra-mitochondrial space as a fraction of 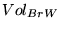 |
8.0000e-02 |
(12) (p182) has the estimates of 10 per cent cell volume for the whole mitochondrion, about 5 per cent for the inner matrix space (which is more important to us). (40) suggests 13 per cent for the matrix, 6 per cent for the intermembrane space. Possible reference - http://www.nsf.gov/pubs/1999/nsf98106/98106htm/tp100.html
for antarctic fishes has values of 15-30 per cent. |
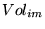 |
Vol_inm |
volume of the intra-mitochondrial space |
8.0000e+01 (fcn) |
|
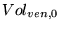 |
Vol_ven00 |
volume of the veins when the transmural pressure difference is zero |
1.4723e+01 (fcn) |
|
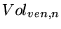 |
Vol_venn |
normal volume of venous blood in tissue |
3.0834e+01 (fcn) |
normal fraction of brain volume which is venous blood is given as 0.0237 in Aubert et al (10). This translates to a normal volume of
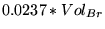 (= about 29). In (11) it is stated that approximately 70-80 per cent of blood volume in the brain is venous. (39) give a value of 50 ml for (= about 29). In (11) it is stated that approximately 70-80 per cent of blood volume in the brain is venous. (39) give a value of 50 ml for 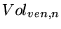 in table 1 but have a higher total blood volume in the brain. in table 1 but have a higher total blood volume in the brain. |
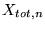 |
X_totn |
normal concentration of haemoglobin in blood |
2.2700e+00 |
12-17 g/dL given in Table IX-1 in (22). Since the atomic weight of Hb is 66 kD, this translates to 1.81 to 2.57 mM |
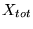 |
X_tot |
concentration of haemoglobin in blood |
2.2700e+00 |
control anaemia with this |
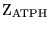 |
Z_ATPH |
The normal number of ATP's produced per proton pumped out the mitochondrion by the electron transport chain |
2.2069e-01 (fcn) |
See section 3. |
![$\ensuremath{\mathrm{[ADP_{cyt}]_n}}$](img557.png) |
_ADPn |
normal ADP concentration in cytoplasm |
3.5000e-01 |
0.2-0.3 mM on p14 of (11). 0.56 in micromoles/g, rats, (12), p226. consistently about 0.25-0.35 of ATP concentrations in (41) for heart muscle. Consistently a lot about 50 to 100 times lower than ATP values in (42). 0.14 mM for erythrocytes, p574, (20). Need to get hold of Roth and Weiner, cited in (10) for some brain values. A range of 0.05 to 0.2 mM is given in (13). Note that inasmuch as this quantity and normal ATP levels determine the mitochondrial levels of these metabolites, and hence the maximal rate of oxidative phosphorylation, we have to be careful to set them such that the normal rate of oxidative phosphorylation is positive... |
![$\ensuremath{\mathrm{[ADP_{im}]_n}}$](img558.png) |
_mADPn |
normal ADP concentration in mitochondria |
8.2677e-01 (fcn) |
This is currently calculated based on the assumption that the total ATP-ADP pool is the same as in the cytoplasm, but the ADP/ATP ratio is
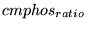 times that in the cytoplasm. (13) assume a lower value of about 1 mM. The lower the normal value that we assume, the more possibility there is that increased demand can upregulate the rate of oxidative phosphorylation. times that in the cytoplasm. (13) assume a lower value of about 1 mM. The lower the normal value that we assume, the more possibility there is that increased demand can upregulate the rate of oxidative phosphorylation. |
![$\ensuremath{\mathrm{[AMP_{cyt}]_n}}$](img560.png) |
_AMPn |
normal AMP concentration in cytoplasm |
1.1367e-01 (fcn) |
0.04 mM on p144 of (11) (presumably for whole cells). 0.4 in micromoles/g, rats, (12), p226, 0.5 mM in (32). These last two values seem too high to be squared with two facts: that the adenylate kinase reaction is in equilibrium, and that ADP concentrations are much lower than ATP concentrations. |
![$\ensuremath{\mathrm{[ATP_{cyt}]_n}}$](img561.png) |
_ATPn |
normal ATP concentration in cytoplasm |
3.0000e+00 |
3.03 (average brain tissue value) given in (43). The value in mM for humans is given as 2.19 and 3.41 for gray and white matter respectively at http://mrrc.aecom.yu.edu/SI31p.htm. 1.85 in mM, erythrocytes, p574, (20). 2.45 in micromoles/g, rats, (12), p226. Assuming that most of the ATP+ADP is in the form of ATP, the value of 6.7 mM for this total in Korzeniewski (5) seems too high. |
![$\ensuremath{\mathrm{[ATP_{im}]_n}}$](img562.png) |
_mATPn |
normal ATP concentration in mitochondria |
1.4173e+01 (fcn) |
this is currently calculated based on the assumption that the total ATP-ADP pool is the same as in the cytoplasm, but the ADP/ATP ratio is
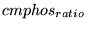 times that in the cytoplasm (about 10.0?) times that in the cytoplasm (about 10.0?) |
![$\ensuremath{\mathrm{[Ad_{cyt}]_n}}$](img563.png) |
_Adn |
normal adenosine concentration in cytoplasm |
1.0000e-05 |
the range 0.02 - 2 micromolar is given on p314 of (11) for extracellular adenosine |
![$\ensuremath{\mathrm{[Ad_{ec}]_n}}$](img564.png) |
_eAdn |
normal adenosine concentration in extracellular space |
1.0000e-05 (fcn) |
same as cytoplasmic value. p14 of Latini (44) has the value of 40-90 nM in CSF and later on p15 180-240 nM under normoxic conditions. The somewhat higher range 0.02 - 2 micromolar is given on p314 of (11) for extracellular adenosine |
![$\ensuremath{\mathrm{[Aden]_{im, tot}}}$](img565.png) |
_mATPADPpool |
mitochondrial concentration of ATP + ADP |
1.5000e+01 |
a value of 15.0 mM is given in Cortassa et al (13) - considerably higher than in the cytoplasm. Korzeniewski (5) has a value of 16.26 mM. A value of 10 mM is implied for myocites in (40). But a glance at http://mrrc.aecom.yu.edu/SI31p.htm
suggests that these values are quite tissue dependent in cytoplasm and hence probably in mitochondria. So for example in (40) it is implied that cytoplasmic ATP level is about 9 mM, although this seems too high for the brain. |
![$\ensuremath{\mathrm{[BiC_a]_n}}$](img566.png) |
BiC_an |
normal arterial bicarbonate ion concentration |
2.4367e+01 (fcn) |
25 in micromoles/ml, rats, (12) - p213 - confirmed elsewhere on internet - confirmed with Ilias |
![$\ensuremath{\mathrm{[BiC_a]}}$](img567.png) |
BiC_a |
arterial bicarbonate ion concentration |
2.4367e+01 (fcn) |
In general there will be some regulation to increased CO2 - see for example (45) - an effect we currently do not take into account. |
![$\ensuremath{\mathrm{[CO_{2, a}]_n}}$](img568.png) |
CO2_an |
normal arterial concentration of dissolved carbon dioxide |
1.2000e+00 (fcn) |
|
![$\ensuremath{\mathrm{[CO_{2, a}]}}$](img569.png) |
CO2_a |
arterial concentration of dissolved carbon dioxide. |
1.2000e+00 (fcn) |
|
![$\ensuremath{\mathrm{[CO_{2, cyt}]_n}}$](img570.png) |
_CO2n |
normal cytoplasmic
 concentration concentration |
2.0520e+00 (fcn) |
|
![$\ensuremath{\mathrm{[CO_{2, c}]_n}}$](img571.png) |
CO2n |
normal capillary
 concentration concentration |
1.5157e+00 (fcn) |
This is the dissolved gas (not including the carbaminohaemoglobin.) |
![$\ensuremath{\mathrm{[CO_{2, ecs}]_n}}$](img572.png) |
_eCO2n |
normal CSF
 concentration concentration |
1.7838e+00 (fcn) |
In rats a value of 1.3 is given in Wang et al (46) |
![$\ensuremath{\mathrm{[CO_{2, im}]_n}}$](img573.png) |
_mCO2n |
normal mitochondrial
 concentration concentration |
2.4624e+00 (fcn) |
|
![$\ensuremath{\mathrm{[CO_{2, v}]_n}}$](img574.png) |
CO2_vn |
normal concentration of dissolved carbon dioxide in venous blood |
1.5300e+00 (fcn) |
|
![$\ensuremath{\mathrm{[Ca_{ecs}^{2+}]}}$](img575.png) |
Ca_en |
normal extracellular calcium concentration |
1.0000e+01 |
2.1 mM in CSF at this site, 1 mM on p160 of (3) |
![$\ensuremath{\mathrm{[Ca_{i, 1}^{2+}]_n}}$](img576.png) |
Ca_i1n |
normal calcium ion concentration in the proximal VSM segment |
1.0000e-04 |
1e-4 in (8) for frog muscle, p47. Same value in (3), p51 and p160. We assume the same in both segments |
![$\ensuremath{\mathrm{[Ca_{i, 2}^{2+}]_n}}$](img577.png) |
Ca_i2n |
normal calcium ion concentration in the distal VSM segment |
1.0000e-04 |
1e-4 in (8) for frog muscle, p47. Same value in (3), p51 and p160. We assume the same in both segments |
![$\ensuremath{\mathrm{[Cr_{cyt}]_n}}$](img578.png) |
_Crn |
normal concentration of creatine in cytoplasm |
3.9000e+00 (fcn) |
|
![$\ensuremath{\mathrm{[Cr_{cyt}]_{n, w}}}$](img579.png) |
_Crnw |
normal creatine concentration in brain tissue in micromoles/g |
3.0000e+00 |
based on the value given in (12), p227, p12 |
![$\ensuremath{\mathrm{[FADH_{2, cyt}]_n}}$](img580.png) |
_mFADHn |
normal levels of
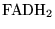 in brain cell mitochondria in brain cell mitochondria |
1.2200e+00 |
(13) have a value of 1.22 mM/L, but no reference for this is quoted. A small size for the FAD pool, and in particular of FADH levels suggests that if oxygen is cut out suddenly, it is FAD which will run out first and bring the TCA cycle to a halt with the NAD/NADH ratio still quite high... |
![$\ensuremath{\mathrm{[FAD_{cyt}]_n}}$](img581.png) |
_mFADn |
normal levels of FAD in brain cell mitochondria |
1.0000e-01 |
(13) have a value of 0.01 mM/L but no reference for this is quoted. A small size for the FAD pool, and in particular of FADH levels suggests that if oxygen is cut out suddenly, it is FAD which will run out first and bring the TCA cycle to a halt with the NAD/NADH ratio still quite high... |
![$\ensuremath{\mathrm{[HCO^-_{3(c)}]_n}}$](img582.png) |
BiCn |
normal capillary bicarbonate ion concentration |
2.5760e+01 (fcn) |
25 in micromoles/ml, rats, (12) - p213 - this is currently very crudely set. The alternative would be to set it according to the equilibrium with
 and H - but this tends to lead to a value which is too large. p50 of Rapoport, human data, gives a value of 26.8. and H - but this tends to lead to a value which is too large. p50 of Rapoport, human data, gives a value of 26.8. |
![$\ensuremath{\mathrm{[HCO^-_{3, cyt}]_n}}$](img583.png) |
_BiCn |
normal cytoplasmic bicarbonate concentration |
1.9046e+01 (fcn) |
calculated on the assumption of equilibrium with CO2 and H+ |
![$\ensuremath{\mathrm{[HCO^-_{3, ec}]_n}}$](img584.png) |
_eBiCn |
normal CSF bicarbonate ion concentration |
2.5060e+01 (fcn) |
p50 of Rapoport - human data - gives a value of 23.3 |
![$\ensuremath{\mathrm{[HCO^-_{3, im}]_n}}$](img585.png) |
_mBiCn |
normal mitochondrial bicarbonate concentration |
9.0988e+01 (fcn) |
calculated on the assumption of equilibrium with CO2 and H+ |
![$\ensuremath{\mathrm{[H^+_a]_n}}$](img586.png) |
Hy_an |
normal arterial hydrogen ion concentration |
3.8905e-05 (fcn) |
calculated from the equilibrium of CO2 and BiC - this is to allow for the possibility of altering CO2 and hence blood pH (i.e. BiC regulation to restore blood pH will not be instantaneous.) |
![$\ensuremath{\mathrm{[H^+_a]}}$](img587.png) |
Hy_a |
arterial hydrogen ion concentration |
3.8905e-05 (fcn) |
calculated from the equilibrium of CO2 and BiC - this is to allow for the possibility of altering CO2 and hence blood pH (i.e. BiC regulation to restore blood pH will not be instantaneous.) |
![$\ensuremath{\mathrm{[H^+_v]_n}}$](img588.png) |
Hy_vn |
normal venous hydrogen ion concentration |
5.0119e-05 (fcn) |
|
![$\ensuremath{\mathrm{[H^+_{cyt}]_n}}$](img589.png) |
_Hyn |
normal hydrogen ion concentration in cytoplasm |
8.5114e-05 (fcn) |
|
![$\ensuremath{\mathrm{[H^+_{c}]_n}}$](img590.png) |
Hyn |
normal hydrogen ion concentration in capillary blood |
4.4157e-05 (fcn) |
|
![$\ensuremath{\mathrm{[H^+_{ec}]_n}}$](img591.png) |
_eHyn |
normal hydrogen ion concentration in extracellular space |
5.6234e-05 (fcn) |
|
![$\ensuremath{\mathrm{[H^+_{im}]_n}}$](img592.png) |
_mHyn |
normal hydrogen ion concentration in mitochondria |
2.1380e-05 (fcn) |
|
![$\ensuremath{\mathrm{[Hb(H^+)_{n_H, a}]_n}}$](img593.png) |
X_H_an |
normal arterial concentration of protonated haemoglobin |
6.3329e-02 (fcn) |
|
![$\ensuremath{\mathrm{[Hb(H^+)_{n_H, a}]}}$](img594.png) |
X_H_a |
arterial concentration of protonated haemoglobin |
6.3329e-02 (fcn) |
|
![$\ensuremath{\mathrm{[Hb(H^+)_{n_H}]_n}}$](img595.png) |
X_Hn |
normal concentration of capillary haemoglobin combined with
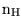 protons protons |
3.1960e-01 (fcn) |
|
![$\ensuremath{\mathrm{[Hb(O_2)_4(H^+)_{n_H}]_n}}$](img597.png) |
X_O4_Hn |
normal concentration of capillary haemoglobin combined with oxygen and protons |
3.3572e-01 (fcn) |
|
![$\ensuremath{\mathrm{[Hb(O_2)_4]_n}}$](img598.png) |
X_O4n |
normal concentration of capillary haemoglobin combined with four oxygen molecules |
1.5206e+00 (fcn) |
|
![$\ensuremath{\mathrm{[Hb(O_2)_{4, a}]_n}}$](img599.png) |
X_O4_an |
normal arterial concentration of oxygenated haemoglobin |
1.8296e+00 (fcn) |
|
![$\ensuremath{\mathrm{[Hb(O_2)_{4, a}]}}$](img600.png) |
X_O4_a |
arterial concentration of oxygenated haemoglobin |
1.8296e+00 (fcn) |
|
![$\ensuremath{\mathrm{[Hb(O_2)_{4, v}]_n}}$](img601.png) |
X_O4_vn |
normal venous concentration of
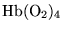 |
7.6644e-01 (fcn) |
|
![$\ensuremath{\mathrm{[Hb(O_2)_{4}(H^+_{n_H, a})]_n}}$](img603.png) |
X_O4_H_an |
normal arterial concentration of oxygenated and protonated haemoglobin |
3.5590e-01 (fcn) |
|
![$\ensuremath{\mathrm{[Hb(O_2)_{4}(H^+_{n_H, a})]}}$](img604.png) |
X_O4_H_a |
arterial concentration of oxygenated and protonated haemoglobin |
3.5590e-01 (fcn) |
|
![$\ensuremath{\mathrm{[HbC]_n}}$](img605.png) |
CYn |
normal concentration of
 -bound Hb amino groups in capillary blood -bound Hb amino groups in capillary blood |
3.0181e+00 (fcn) |
|
![$\ensuremath{\mathrm{[HbC_a]_n}}$](img606.png) |
CY_an |
normal concentration of
 -bound Hb amino groups in arterial blood. -bound Hb amino groups in arterial blood. |
3.0000e+00 (fcn) |
|
![$\ensuremath{\mathrm{[HbC_a]}}$](img607.png) |
CY_a |
concentration of
 -bound Hb amino groups in arterial blood -bound Hb amino groups in arterial blood |
3.0000e+00 (fcn) |
|
![$\ensuremath{\mathrm{[Hb]_n}}$](img608.png) |
Xn |
normal concentration of capillary haemoglobin (not combined with oxygen or protons) |
9.4093e-02 (fcn) |
|
![$\ensuremath{\mathrm{[Hb_a]_n}}$](img609.png) |
X_an |
normal arterial concentration of haemoglobin |
2.1162e-02 (fcn) |
|
![$\ensuremath{\mathrm{[Hb_a]}}$](img610.png) |
X_a |
arterial concentration of haemoglobin (not bound to oxygen or protons) |
2.1162e-02 (fcn) |
|
![$\ensuremath{\mathrm{[Hb_o]_n}}$](img611.png) |
Yn |
normal concentration of unbound Hb amino groups in capillary blood |
6.0619e+00 (fcn) |
|
![$\ensuremath{\mathrm{[Hb_v]_n}}$](img612.png) |
X_vn |
normal venous concentration of
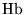 |
1.5036e+00 (fcn) |
|
![$\ensuremath{\mathrm{[Hb_{o, a}]_n}}$](img614.png) |
Y_an |
normal concentration of unbound Hb amino groups in arterial blood. |
6.0800e+00 (fcn) |
|
![$\ensuremath{\mathrm{[Hb_{o, a}]}}$](img615.png) |
Y_a |
concentration of unbound Hb amino groups in arterial blood. |
6.0800e+00 (fcn) |
|
![$\ensuremath{\mathrm{[K^+_a]}}$](img616.png) |
K_a |
arterial potassium ion concentration |
4.0700e+00 |
We set this to be very slightly higher than we want the brain concentration to be because transport from the blood must compensate outflow in CSF. |
![$\ensuremath{\mathrm{[K^+_{cyt}]_n}}$](img617.png) |
_Kn |
normal concentration of potassium ions in cytoplasm |
1.8000e+02 (fcn) |
in Edvinsson et al. CBFM (old ed.) a value of 180 mM is given for the cat MCA |
![$\ensuremath{\mathrm{[K^+_{c}]_n}}$](img618.png) |
Kn |
normal capillary concentration of potassium |
4.0000e+00 |
|
![$\ensuremath{\mathrm{[K^+_{ecs}]_n}}$](img619.png) |
_eKn |
normal concentration of potassium ions in extracellular space |
2.8000e+00 |
2.8 mM in CSF at this site. |
![$\ensuremath{\mathrm{[K^+_{mus}]_n}}$](img620.png) |
_K_musn |
normal potassium ion concentration in VSM cells |
1.8000e+02 (fcn) |
|
![$\ensuremath{\mathrm{[L_a]}}$](img621.png) |
L_a |
arterial lactic acid concentration |
2.8192e-04 (fcn) |
|
![$\ensuremath{\mathrm{[MLC_{1}]_n}}$](img622.png) |
MLC1n |
normal concentration of dephosphorylated myosin heads in the VSM of the proximal arterial segment |
4.4800e-02 (fcn) |
|
![$\ensuremath{\mathrm{[MLC_{2}]_n}}$](img623.png) |
MLC2n |
normal concentration of dephosphorylated myosin heads in the VSM of the distal arterial segment |
4.7600e-02 (fcn) |
|
![$\ensuremath{\mathrm{[MLC_{p, 1}]_n}}$](img624.png) |
MLCp1n |
normal concentration of phosphorylated myosin heads in the VSM of the proximal arterial segment |
1.1200e-02 (fcn) |
|
![$\ensuremath{\mathrm{[MLC_{p, 2}]_n}}$](img625.png) |
MLCp2n |
normal concentration of phosphorylated myosin heads in the VSM of the distal arterial segment |
8.4000e-03 (fcn) |
|
![$\ensuremath{\mathrm{[NADH_{cyt}]_n}}$](img626.png) |
_NADHn |
normal cytoplasmic NADH concentration. |
2.9703e-03 (fcn) |
On the assumption that the lactate dehydrogenase reaction is approximately in equilibrium, and that the ratio of NAD/NADH is
![$2.63e04\ensuremath{\mathrm{[H^+]}}\ensuremath{\mathrm{[Py^-]}}/\ensuremath{\mathrm{[lac^-]}}$](img627.png) we get different values. In (20), p612, an NAD/NADH ratio of 20 is given, which is very different from that calculated from p574. (22), p264 has a cytoplasmic ratio of about 1000:1. Aubert et al (10) have an intracellular NADH value of 0.026 mM which is presumably a weighted average of cytoplasmic and mitochondrial (even though the mitochondria are much smaller they probably contain the majority of reduced NADH in the cell). we get different values. In (20), p612, an NAD/NADH ratio of 20 is given, which is very different from that calculated from p574. (22), p264 has a cytoplasmic ratio of about 1000:1. Aubert et al (10) have an intracellular NADH value of 0.026 mM which is presumably a weighted average of cytoplasmic and mitochondrial (even though the mitochondria are much smaller they probably contain the majority of reduced NADH in the cell). |
![$\ensuremath{\mathrm{[NADH_{im}]_n}}$](img628.png) |
_mNADHn |
normal mitochondrial NADH concentration. |
9.0909e-01 (fcn) |
See also (20) p 574. On the assumption that the lactate dehydrogenase reaction is approximately in equilibrium, and that the ratio of NAD/NADH is
![$2.63e04\ensuremath{\mathrm{[H^+]}}\ensuremath{\mathrm{[Py^-]}}/\ensuremath{\mathrm{[lac^-]}}$](img627.png) we get different values. In (20), p612, an NAD/NADH ratio of 20 is given, which is very different from that calculated from p574. This website
has a mitochondrial ratio of about 10/1 we get different values. In (20), p612, an NAD/NADH ratio of 20 is given, which is very different from that calculated from p574. This website
has a mitochondrial ratio of about 10/1 |
![$\ensuremath{\mathrm{[NAD]_{cyt, tot}}}$](img629.png) |
_NADpool |
total NAD pool in cytoplasm |
3.0000e-01 |
See section 4.2. In (7) only about 517 +- 23 micromoles/kg for whole brain in mice. This translates to about 0.64 mM on average. We assume a somewhat higher value in humans. Then we can calculate
![$\ensuremath{\mathrm{[NAD]_{im, tot}}}$](img630.png) and and
![$\ensuremath{\mathrm{[NAD]_{im, tot}}}$](img630.png) . . |
![$\ensuremath{\mathrm{[NAD]_{im, tot}}}$](img630.png) |
_mNADpool |
total NAD pool in mitochondria |
1.0000e+01 |
In Cortassa et al (13) a value of 10.0 mM is given with a reference to Albe. This is approximately consistent with an average cellular content of about 0.85 mM NAD and the assertion in (6) that about 72 per cent of NAD is in the mitochondria. The lower value of 2.97 mM is given in Korzeniewski (5). |
![$\ensuremath{\mathrm{[NAD_{cyt}]_n}}$](img631.png) |
_NADn |
normal concentration of NAD in cytoplasm |
2.9703e-01 (fcn) |
See also (20) p 574. On the assumption that the lactate dehydrogenase reaction is approximately in equilibrium, and that the ratio of Nad/Nadh is
![$2.63e04\ensuremath{\mathrm{[H^+]}}\ensuremath{\mathrm{[Py^-]}}/\ensuremath{\mathrm{[lac^-]}}$](img627.png) we get different values. In (20), p612, an NAD/NADH ratio of 20 is given, which is very different from that calculated from p574. (22), p264 has a cytoplasmic ratio of about 1000:1 we get different values. In (20), p612, an NAD/NADH ratio of 20 is given, which is very different from that calculated from p574. (22), p264 has a cytoplasmic ratio of about 1000:1 |
![$\ensuremath{\mathrm{[NAD_{im}]_n}}$](img632.png) |
_mNADn |
normal concentration of NAD in mitochondria |
9.0909e+00 (fcn) |
see also (20) p 574. On the assumption that the lactate dehydrogenase reaction is approximately in equilibrium, and that the ratio of
![$2.63e04\ensuremath{\mathrm{[H^+]}}\ensuremath{\mathrm{[Py^-]}}/\ensuremath{\mathrm{[lac^-]}}$](img627.png) we get different values. In (20), p612, an NAD/NADH ratio of 20 is given, which is very different from that calculated from p574. This website
has a mitochondrial ratio of about 10/1 we get different values. In (20), p612, an NAD/NADH ratio of 20 is given, which is very different from that calculated from p574. This website
has a mitochondrial ratio of about 10/1 |
![$\ensuremath{\mathrm{[NO_1]_n}}$](img633.png) |
NO1n |
normal concentration of NO in the proximal VSM segment |
2.0000e-06 |
the half maximal concentration for activation of cGMP seems to be about 4 nM in (18), suggesting normal concentrations of less than this, otherwise increases in NO would have no effect. 1 micromolar in a stimulated endothelial cell in vitro in (47) p3, but this is almost certainly a gross overestimate. |
![$\ensuremath{\mathrm{[NO_2]_n}}$](img634.png) |
NO2n |
normal concentration of NO in the distal VSM segment |
2.0000e-06 |
the half maximal concentration for activation of cGMP seems to be about 4 nM in (18), suggesting normal concentrations of less than this, otherwise increases in NO would have no effect. 1 micromolar in a stimulated endothelial cell in vitro in (47) p3, but this is almost certainly a gross overestimate. |
![$\ensuremath{\mathrm{[Na^+_a]}}$](img635.png) |
Na_a |
arterial sodium ion concentration. |
1.3800e+02 |
Control parameter. Normally set to value of
![$\ensuremath{\mathrm{[Na^+_a]_n}}$](img636.png) |
![$\ensuremath{\mathrm{[Na^+_{cyt}]_n}}$](img637.png) |
_Nan |
normal concentration of sodium ions in cytoplasm |
1.5000e+01 |
5-15 mM at this site, 15.0 in Aubert et al (10), 10.0 in (13) (heart) |
![$\ensuremath{\mathrm{[Na^+_{c}]_n}}$](img638.png) |
Nan |
normal concentration of sodium ions in capillary blood |
1.3800e+02 |
Since there is no net production or removal in the brain in the model we can assume that this is the same as the arterial value |
![$\ensuremath{\mathrm{[Na^+_{ecs}]_n}}$](img639.png) |
_eNan |
normal concentration of sodium ions in extracellular space |
1.3800e+02 |
138 mM in CSF at this site |
![$\ensuremath{\mathrm{[O_{2, a}]_n}}$](img640.png) |
O2_an |
normal concentration of dissolved oxygen in arterial blood |
1.3300e-01 (fcn) |
|
![$\ensuremath{\mathrm{[O_{2, a}]}}$](img641.png) |
O2_a |
concentration of dissolved oxygen in arterial blood |
1.3300e-01 (fcn) |
|
![$\ensuremath{\mathrm{[O_{2, cyt}]_n}}$](img642.png) |
_O2n |
normal concentration of dissolved oxygen in cytoplasm |
4.5500e-02 (fcn) |
In Aubert et al (10) a value of 0.0262 mM is given. This could be an average over cytoplasm and mitochondria, or their estimate of mitochondrial concentration since this is the site of use... |
![$\ensuremath{\mathrm{[O_{2, cyt}]_{nw}}}$](img643.png) |
_O2nw |
normal concentration of oxygen per unit weight of brain tissue |
3.5000e-02 |
0.045 micromoles/g - p232 (12) - so
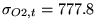 |
![$\ensuremath{\mathrm{[O_{2, c}]_n}}$](img645.png) |
O2n |
normal concentration of dissolved oxygen in capillary blood |
6.8000e-02 (fcn) |
This parameter has an important influence on the dynamics. In fact it should be derivable from delivery criteria alone, but can also be set by trial and error. It should be changed in tandem with CMRgluc (and hence CMRO2) |
![$\ensuremath{\mathrm{[O_{2, ecs}]_n}}$](img646.png) |
_eO2n |
normal CSF
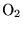 concentration concentration |
5.0000e-02 (fcn) |
In rats a value of 0.068 mM/L is given in Wang et al (46). Data in (48) has a tissue pO2 value of about 15 mmHg, suggesting a concentration of about value of about 0.02 mM/L if the solubility is much the same as in blood. |
![$\ensuremath{\mathrm{[O_{2, im}]_n}}$](img647.png) |
_mO2n |
normal concentration of dissolved oxygen in mitochondria |
2.2750e-02 (fcn) |
This parameter is important. If it is low then as blood O2 drops (e.g. if blood flow drops), then diffusion between blood and mitochondria can become limiting for mitochondrial respiration. When this happens depends on the normal gradient between the two. On p93 of (7) it is stated that brain cells are normally exposed to oxygen pressures lower than those required to saturate the mitochondrial respiratory chain, suggesting oxygen tensions of less than about 10 mmHg. In (18) the range of 20-30 micromoles is given. In Aubert et al (10) a value of 0.0262 mM is given. This could be an average over cytoplasm and mitochondria, or their estimate of mitochondrial concentration since this is the site of use... |
![$\ensuremath{\mathrm{[O_{2, v}]_n}}$](img648.png) |
O2_vn |
normal concentration of dissolved oxygen in venous blood |
5.0120e-02 (fcn) |
|
![$\ensuremath{\mathrm{[Ox_{im}]_n}}$](img649.png) |
_Oxn |
normal concetration of oxaloacetate in mitochondria |
5.4000e-01 |
107 +- 25 nanomoles/g wet weight in human VSM in (49). This translates to about 0.1 mM if oxaloacetate is evenly distributed in both cytoplasm and mitochondria and about 2.0 mM if it is just distributed in the mitochondria. Although we know that OAA exists in both compartments, we have no idea of it's relative compartmentation... |
![$\ensuremath{\mathrm{[PCr_{cyt}]_n}}$](img650.png) |
_PCrn |
normal concentration of phosphocreatine (creatine phosphate) in cell cytoplasm |
5.8500e+00 (fcn) |
(10) is held as biochemodel/aubert.pdf |
![$\ensuremath{\mathrm{[PCr_{cyt}]_{n, w}}}$](img651.png) |
_PCrnw |
normal phosphocreatine (creatine phosphate) concentration in brain tissue per unit weight |
4.5000e+00 |
4.5 in (12), p227, p12 |
![$\ensuremath{\mathrm{[Phos_{cyt}]_n}}$](img652.png) |
_Phosn |
normal inorganic phosphate concentration in cytoplasm |
1.5000e+00 |
(43) has a value of about 1.5 mM. A value of 3 mM is given in (40) for the cytoplasm in myocytes. In (2), a CSF value of 34 mg/L is given which is equivalent to just over 1 mM/L as the molecular weight of phosphorous is 30.97 |
![$\ensuremath{\mathrm{[Phos_{im}]_n}}$](img653.png) |
_mPhosn |
normal phosphate concentration in mitochondria |
3.2000e+00 |
a value of about 6.75 mM (or a little over twice the cytoplasmic value) is implied by equation 15 in (40) for myocytes. If we assume that this doubling is also true in the brain, we get a value of about 3.2 mM. In (13) the much higher value of 20.0 mM is given for myocytes. |
![$\ensuremath{\mathrm{[Py^-_{cyt}]_n}}$](img654.png) |
_Py0n |
normal pyruvate ion concentration in the cytoplasm |
1.2000e-01 |
(12), p228, 0.118 in micromoles/g (for rats). (20) p574, 0.051 mM in erythrocytes. Aubert - 0.16 mM (human brain) for intracellular pyruvate. Presumably all of these values are weighted averages of cytoplasmic and mitochondrial pyruvate. 0.09 micromoles/gram given for whole rat brain on p144 of (11) which translates to 0.117 mM average in brain wet matter. |
![$\ensuremath{\mathrm{[Py^-_{ecs}]_n}}$](img655.png) |
_ePy0n |
normal pyruvate ion concentration in the extracellular space |
1.2000e-01 (fcn) |
(12), rats, p228, 0.118 in micromoles/g |
![$\ensuremath{\mathrm{[Py^-_{im}]_n}}$](img656.png) |
_mPy0n |
normal mitochondrial pyruvate ion concentration |
3.8218e-01 (fcn) |
a guess at the moment |
![$\ensuremath{\mathrm{[\alpha KG_{im}]_n}}$](img657.png) |
_AKGn |
normal concentration of
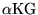 in mitochondria in mitochondria |
1.0000e-01 |
the ball park figures of 0.1-2 mM are implied by figure A3 in (13). The value of 0.07+- 0.04 micromoles per gram dry weight for the heart is given in (50). The fact that alpha-ketoglutarate takes part in the malate-aspartate shuttle suggests it is not localised in mitochondria. If we assume a brain water content is 80 per cent, and the same distribution in cytoplasm and mitochondria, we get a concentration of about 0.0175 mM. The value of 0.11 mM/kg wet weight is given for immature rat brains in (23) which implies a value of about 0.132 mM which is significantly higher than the value for the heart. |
![$\ensuremath{\mathrm{[gluc]_n}}$](img658.png) |
_glucn |
normal levels of glucose in brain cell cytoplasm |
1.4000e+00 |
1.2 mM given in Aubert et al (10). 1.6 micromoles/gram given for whole rat brain on p144 of (11) which translates to about 2.1 mM average in brain wet matter. |
![$\ensuremath{\mathrm{[gluc_a]_n}}$](img659.png) |
gluc_an |
normal arterial glucose concentration |
5.1100e+00 |
3.9-6.1 is given in Table IX-I in (22). A value of 90 mg/dL is given at this site, which translates to 5 mM (molecular weight of glucose is 180) |
![$\ensuremath{\mathrm{[gluc_a]}}$](img660.png) |
gluc_a |
arterial glucose concentration |
5.1100e+00 |
control hypoglycemia with this |
![$\ensuremath{\mathrm{[gluc_c]_n}}$](img661.png) |
glucn |
normal concentration of glucose in capillary plasma |
4.8396e+00 (fcn) |
5.64 +- 0.39 mM in humans in arterial blood under basal fasting conditions in (51), Aubert et al (10) give a value of 4.56 mM in capillary blood |
![$\ensuremath{\mathrm{[gluc_v]_n}}$](img662.png) |
gluc_vn |
normal glucose concentration in veins |
2.0000e+00 |
guess - just set to the tissue values |
![$\ensuremath{\mathrm{[gluc_{ecs}]_n}}$](img663.png) |
_eglucn |
normal concentration of glucose in extracellular fluid |
2.0879e+00 (fcn) |
0.82 +- 0.27 mM in humans under basal fasting conditions in (51). (12), rats, whole brain, p228, 1.37 in micromoles/g |
![$\ensuremath{\mathrm{[lac^-_a]}}$](img664.png) |
L0_a |
arterial lactate concentration |
1.0000e+00 |
|
![$\ensuremath{\mathrm{[lac^-_c]_n}}$](img665.png) |
L0n |
normal levels of lactate in capillaries |
1.0200e+00 |
Bhagavan has an interval of 0.5-2.0 mM for arterial blood. We assume that it is lower than the tissue values - i.e. that there is some gradient for outflow. 0.96 +- 0.2 mM in (51) 0.35 mM in (10) in capillary blood |
![$\ensuremath{\mathrm{[lac^-_{cyt}]_n}}$](img666.png) |
_L0n |
normal cytoplasmic concetration of lactate |
2.0000e+00 |
(12), rats, p228: 1.39 micromoles/g; (20), p574, erythrocytes, 2.9 mM; In Aubert et al. (10) a value of 1 mM is used |
![$\ensuremath{\mathrm{[lac^-_{ecs}]_n}}$](img667.png) |
_eL0n |
Normal levels of lactate in ECF |
1.9020e+00 (fcn) |
1.38 +- 1.2 mM in human under basal fasting conditions (51). Note the large variance. |
![$\ensuremath{\mathrm{[lac_{cyt}]_n}}$](img668.png) |
_lacn |
normal concentration of undissociated lactic acid in cytoplasm |
1.2335e-03 (fcn) |
|
![$\ensuremath{\mathrm{[lac_{c}]_n}}$](img669.png) |
Ln |
normal concentration of undissociated lactic acid in capillary blood |
3.2638e-04 (fcn) |
|
![$\ensuremath{\mathrm{[lac_{ecs}]_n}}$](img670.png) |
_eLn |
normal concentration of undissociated lactic acid in extracellular space |
7.7505e-04 (fcn) |
|
![$\ensuremath{\mathrm{[suc_{cyt}]_n}}$](img671.png) |
_sucn |
normal concentration of succinate in mitochondria |
1.0000e-01 |
(50) have a value of 0.25 +- 0.07 micromoles/g dry weight for the rat heart. (52) has a value of 0.52 +- 12 micromoles/g dry weight for resting human muscle. If we assume a brain water content is 80 per cent, and that succinate is only distributed in the mitochondria then we get a concentration of about 1.25 mM/2.5 mM. If succinate is distributed evenly throughout the cell, then we get a concentration of about 0.06/0.13 mM |
 |
PD_mitn |
normal membrane potential for the mitochondrial inner membrane |
1.5000e+02 |
150 mV on this site. |
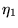 |
eta_1 |
wall viscosity in the proximal arterial segment |
2.3200e+02 |
 in (14) in (14) |
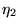 |
eta_2 |
wall viscosity in the distal arterial segment |
4.7800e+01 |
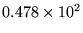 in (14) in (14) |
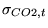 |
henry_CO2t |
solubility of
 in tissue in tissue |
3.8000e-02 |
a total guess - higher than blood? |
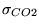 |
henry_CO2 |
solubility of
 in blood in blood |
3.0000e-02 |
0.033 in (3), p481. Some general background on solubilities of gases in liquids at this website |
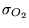 |
henry_O2 |
solubility of oxygen in blood |
1.4000e-03 |
0.0014 in (3), p481. Some general background on solubilities of gases in liquids at this website |
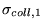 |
sigma_coll1 |
a parameter in the relationship between radius of, and elastic stress in, the vessel walls of the proximal arterial segment |
6.2790e+01 |
62.79 mmHg in (14). Permits the vessels to sustain a moderate negative tension before collapsing. |
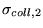 |
sigma_coll2 |
a parameter in the relationship between radius of, and elastic stress in, the vessel walls of the distal arterial segment |
4.1320e+01 |
41.32 mmHg in (14). Permits the vessels to sustain a moderate negative tension before collapsing. |
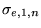 |
sigma_e1n |
normal elastic stress in the vessel walls of the proximal arterial segment |
-2.1716e+01 (fcn) |
|
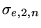 |
sigma_e2n |
normal elastic stress in the vessel walls of the distal arterial segment |
-4.3339e+01 (fcn) |
|
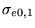 |
sigma_e01 |
a parameter in the relationship between radius of, and elastic stress in, the walls of the proximal arterial segment |
1.4250e-01 |
0.1425 mmHg in (14) |
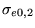 |
sigma_e02 |
a parameter in the relationship between radius of, and elastic stress in, the walls of the distal arterial segment |
1.1190e+01 |
11.19 mmHg in (14) |
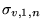 |
sigma_v1n |
normal viscous stress in the vessel walls of the proximal arterial segment |
0.0000e+00 |
|
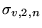 |
sigma_v2n |
normal viscous stress in the vessel walls of the distal arterial segment |
0.0000e+00 |
|
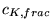 |
c_K_frac |
the basal rate of opening of inward rectifier potassium channels in the absence of exracellular potassium as a fraction of the total normal opening rate |
2.0000e-01 |
See section 5.3. To get vasodilation with addition of potassium over a reasonable range we have to assume this to be small. |
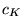 |
c_K |
a parameter describing the basal rate of opening of inward rectifier potassium channels in the absence of exracellular potassium |
1.4099e-03 (fcn) |
See section 5.3 |
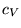 |
c_V |
a parameter representing baseline activation of calcium-sensitive potassium channels in the absence of NO, calcium or a transmural pressure difference |
7.0000e-01 (fcn) |
The fraction of basal channel activation that is contributed by the membrane potential |
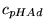 |
c_pHAd |
a parameter describing the baseline activity of ATP-sensitive potassium channels in the absence of protons or adenosine |
3.0030e-04 (fcn) |
See section 5.3. |
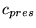 |
c_pres |
a parameter in the relationship between the transmural pressure and the conductivity of calcium-sensitive potassium channels |
9.9990e+03 (fcn) |
See section 5.3 |
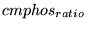 |
cmphos_ratio |
ratio (ATP/ADP)/(mATP/mADP) |
5.0000e-01 |
given as 10 on p186 of (12) but we don't trust this. For the simple model of the ANT to work properly we require a value less than 1. Also (40) have that the mitochondrial ATP:ADP ratio is about 3:1, similar to the cytoplasm |
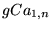 |
gCa1n |
normal conductivity of calcium channels in the proximal VSM segment |
6.7999e-08 (fcn) |
See section 5.4 |
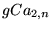 |
gCa2n |
normal conductivity of calcium channels in the distal VSM segment |
6.7013e-08 (fcn) |
See section 5.4 |
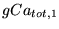 |
gCa1_tot |
total possible calcium channel conductance in the proximal VSM segment |
1.6043e-06 (fcn) |
See section 5.4 |
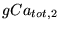 |
gCa2_tot |
total possible calcium channel conductance in the distal VSM arterial segment |
2.2219e-06 (fcn) |
See section 5.4 |
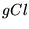 |
gCl |
conductivity of the membrane of VSM cells to chloride ions. |
2.0000e-02 |
a guess - small fraction of gK? |
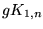 |
gK1n |
normal potassium channel conductivity in the proximal VSM segment |
3.9161e-02 (fcn) |
|
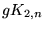 |
gK2n |
normal potassium channel conductivity in the distal VSM segment |
4.3235e-02 (fcn) |
|
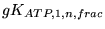 |
gKATP1n_frac |
fraction of normal conductivity contributed by ATP sensitive channels in the proximal VSM segment |
2.0000e-01 |
See section 5.1. in (53) it is stated that these channels don't contribute significantly to basal tone. But according to (33) pC802 the value is about 0.35 - a significant contribution |
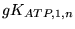 |
gKATP1n |
normal conductivity of ATP sensitive channels in the proximal VSM segment |
7.8321e-03 (fcn) |
See section 5.1. |
 |
gKATP1tot |
total possible conductivity of ATP sensitive potassium channels in the proximal VSM segment |
7.8321e+00 (fcn) |
See section 5.3. |
 |
gKATP2n_frac |
fraction of normal conductivity contributed by ATP sensitive channels in the distal VSM segment |
3.0000e-01 |
See section 5.1. In (53) it is stated that these channels don't contribute significantly to basal tone. But according to (33) pC802 the value is about 0.35 - a significant contribution |
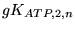 |
gKATP2n |
normal conductivity of ATP sensitive channels in the distal VSM segment |
1.2971e-02 (fcn) |
See section 5.1. |
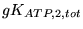 |
gKATP2tot |
total possible conductivity of ATP sensitive potassium channels in the distal VSM segment |
1.2971e+01 (fcn) |
See section 5.3. |
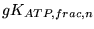 |
gKATPfracn |
the fraction of normally open ATP-sensitive potassium channels - assumed to be the same in each segment |
1.0000e-03 |
We can assume that this is the same for both segments because the extracellular environment is the same for both segments. (33) has the following information: single channel conductance is about 15 - 35 pS; there are 300-500 channels per cell; normal whole cell ATP-sensitive conductance is about 25 pS. these values give a maximum value of about 0.005 for this parameter. In any case, the model does not appear to be very sensitive to this parameter for values less than about 0.2 |
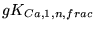 |
gKpres1n_frac |
the fraction of normal potassium channel conductivity contributed by calcium-sensitive potassium channels in the proximal VSM segment |
5.0000e-01 |
See section 5.1. In (53) it is stated that these channels contribute significantly to basal tone in the larger arteries, but not necessarily in the smaller arteries. But (33), pC802 implies that calcium sensitive + IR channels together contribute about a third of resting conductivity |
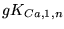 |
gKpres1n |
the normal conductivity of calcium-sensitive potassium channels in the proximal VSM segment |
1.9580e-02 (fcn) |
See section 5.1 |
 |
gKpres2n_frac |
the fraction of normal potassium channel conductivity contributed by calcium-sensitive potassium channels in the distal VSM segment |
4.0000e-01 |
See section 5.1. In (53) it is stated that these channels contribute significantly to basal tone in the larger arteries, but not necessarily in the smaller arteries. But (33), pC802 implies that calcium sensitive + IR channels together contribute about a third of resting conductivity. In (54) it is stated that these channels are quiet in resting conditions in the microcirculation. |
 |
gKpres2n |
the normal conductivity of calcium-sensitive potassium channels in the distal VSM segment |
1.7294e-02 (fcn) |
See section 5.1 |
 |
gKpresfracn |
the fraction of normally open calcium-sensitive potassium channels in each VSM segment |
1.0000e-04 |
in (33) the following information is given: single channel conductance is about 250 pS; 1000-10000 channels per cell; normal conductance of these channels at -60 mV is less than 20 pS. This suggests a value for this parameter of less than 1e-04. The model does not seem to be sensitive to fluctuations in this parameter in this range. |
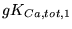 |
gKpres1tot |
total possible conductivity of calcium-sensitive potassium channels in the proximal VSM segment |
1.9580e+02 (fcn) |
See section 5.3 |
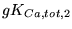 |
gKpres2tot |
total possible conductivity of calcium-sensitive potassium channels in the distal VSM segment |
1.7294e+02 (fcn) |
See section 5.3 |
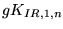 |
gKpot1n |
normal conductivity of inward rectifier potassium channels in the proximal VSM segment |
7.8321e-03 (fcn) |
See section 5.1. (33), pC802 implies that calcium sensitive + IR channels together contribute about a third of resting conductivity. On pC812 it is stated that in proximal segments KIR contribute more to resting membrane potential than in distal segments. In (34) it is stated that these channels contribute to basal tone. In (55) it is stated that these channels do contribute to resting tone in the rat MCA which dilates by up to 8 per cent in response to their blockage. However in (54) the evidence is reviewed and is at best inconclusive. |
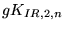 |
gKpot2n |
normal conductivity of inward rectifier potassium channels in the distal VSM segment |
8.6470e-03 (fcn) |
See section 5.1. (33), pC802 implies that calcium sensitive + IR channels together contribute about a third of resting conductivity. On pC812 it is stated that in proximal segments KIR contribute more to resting membrane potential than in distal segments. In (34) it is stated that these channels contribute to basal tone. However in (54) the evidence is reviewed and is at best inconclusive. |
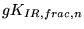 |
gKpotfracn |
the fraction of normally open inward rectifier potassium channels in each segment |
7.0000e-03 |
in (33) we have that: outward channel current is about 1 pA; there are 100-500 inward rectifier potassium channels per cell; normal whole cell current is about 2 pA of which KIR contributes no more than 30 per cent (probably a lot less). These data combine to give a maximum value of about 0.007 for the normally open fraction |
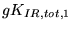 |
gKpot1tot |
total possible conductivity of potassium sensitive potassium channels in the proximal VSM segment |
1.1189e+00 (fcn) |
See section 5.3. |
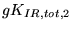 |
gKpot2tot |
total possible conductivity of potassium sensitive potassium channels in the distal VSM segment |
1.2353e+00 (fcn) |
See section 5.3. |
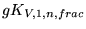 |
gKV1n_frac |
the fraction of normal potassium channel conductivity contributed by voltage gated potassium channels in the proximal VSM segment |
1.0000e-01 |
See section 5.1. According to pC802 of (33) this is about 0.35 i.e. very significant |
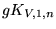 |
gKV1n |
normal value of the conductivity of the voltage gated potassium channels in the proximal VSM segment |
3.9161e-03 (fcn) |
See section 5.1 |
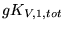 |
gKV1tot |
total possible conductivity of voltage gated potassium channels in the proximal VSM segment |
1.9225e-01 (fcn) |
See section 5.3 |
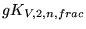 |
gKV2n_frac |
the fraction of normal potassium channel conductivity contributed by voltage gated potassium channels in the distal VSM segment |
1.0000e-01 |
See section 5.1. According to pC802 of (33) this is about 0.35 i.e. very significant |
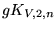 |
gKV2n |
normal value of the conductivity of the voltage gated potassium channels in the distal VSM segment |
4.3235e-03 (fcn) |
See section 5.1 |
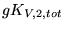 |
gKV2tot |
total possible conductivity of voltage gated potassium channels in the distal VSM segment |
2.6571e-01 (fcn) |
See section 5.3 |
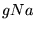 |
gNa |
conductivity of sodium channels in the membranes of VSM cells. |
2.0000e-02 |
(8): only about 3 per cent of gK at rest; (3), p63, 0.01 mS/cm2 - but we want these values at physiological membrane potentials |
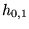 |
h_01 |
a particular wall thickness of the proximal arteries |
3.0000e-03 |
0.003 cm in (14) (not the same as normal wall thickness 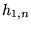 ) ) |
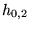 |
h_02 |
a particular wall thickness of the distal arteries |
2.5000e-03 |
0.0025 cm in (14) (not the same as normal wall thickness 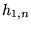 ) ) |
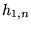 |
h_1n |
normal vessel wall thickness in the proximal arterial segment |
2.0195e-03 (fcn) |
|
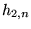 |
h_2n |
normal vessel wall thickness in the distal arterial segment |
2.5854e-03 (fcn) |
|
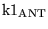 |
k1_ANT |
a constant in the expression for the rate of adenine nucleotide transport by the adenine nucleotide transporter |
8.0000e-01 |
calculated from (13) |
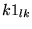 |
k1_lk |
a parameter in the relationship between the proton motive force and the proton leak |
6.2785e-02 (fcn) |
see |
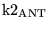 |
k2_ANT |
a constant in the expression for the rate of adenine nucleotide transport by the adenine nucleotide transporter |
1.1110e-01 |
from (13) on the assumption that
 . . |
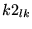 |
k2_lk |
a parameter in the relationship between the proton motive force and the proton leak |
3.8000e-02 |
0.038 per mV given in (5) |
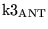 |
k3_ANT |
a constant in the expression for the rate of adenine nucleotide transport by the adenine nucleotide transporter |
7.2000e+00 |
from (13) |
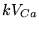 |
kV_Ca |
a parameter in the relationship between membrane potential and the probability of a calcium channel being open |
8.5000e+00 |
Taken from (35) (there called 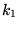 ). Has an important influence on the shape of autoregulation curves. Smaller values give flatter autoregulation curves because of a steeper change in calcium channel conductivity in response to a given change in membrane potential ). Has an important influence on the shape of autoregulation curves. Smaller values give flatter autoregulation curves because of a steeper change in calcium channel conductivity in response to a given change in membrane potential |
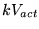 |
kV_act |
a parameter in the relationship between membrane potential and the probability of a voltage-gated potassium channel being open |
1.1000e+01 |
11 mV given in (33); 7.4 mV given for rat VSM in (36) |
 |
kV_gKCa |
a parameter expressing the sensitivity of calcium-sensitive potassium channels to changes in membrane potential |
1.3000e+01 |
pC807 of (33) has the range 12-14 mV |
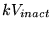 |
kV_inact |
a parameter in the relationship between membrane potential and the probability of a voltage-gated potassium channel being open |
8.0000e+00 |
range from 5 to 11 mV given in (33); -8.8 mV given for rat VSM in (36) |
 |
k_E |
a parameter in the relationship between intracranial compliance and intracranial pressure |
1.1000e-01 |
0.11 in (14) |
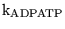 |
k_ADPATP |
the forward rate constant for the conversion of two molecules of ADP to one of ATP and one of AMP |
1.0550e+03 |
Calculated from (5). See Section 4.1 |
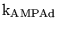 |
k_AMPAd |
the rate of degradation of AMP to adenosine |
1.2195e-06 (fcn) |
methodology of Section 2.2 |
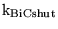 |
k_BiCshut |
the rate of transfer of bicarbonate ions from cytoplasm to mitochondria |
7.8660e-02 (fcn) |
|
 |
k_Bohr |
the ratio of the equilibrium constants for the combination of oxygenated and de-oxygenated haemoglobin with protons |
6.5000e-02 (fcn) |
gives rise to the Bohr shift. Currently calculated from the relative pK values given on this site |
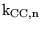 |
k_CCn |
normal rate of transfer of
 across the blood brain barrier across the blood brain barrier |
1.4713e+02 (fcn) |
|
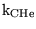 |
k_CHe |
the rate of conversion of
 to bicarbonate and protons in exracellular space to bicarbonate and protons in exracellular space |
5.8826e-02 (fcn) |
Methodology of Section 2.2.4 |
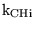 |
k_CHi |
the rate of conversion of
 to bicarbonate and protons in cytoplasm to bicarbonate and protons in cytoplasm |
1.9554e-01 (fcn) |
Methodology of Section 2.2.4 |
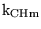 |
k_CHm |
the rate of conversion of
 to bicarbonate and protons in mitochondria to bicarbonate and protons in mitochondria |
2.6995e-02 (fcn) |
Methodology of Section 2.2.4 |
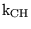 |
k_CH |
the rate of conversion of
 to bicarbonate and protons in blood to bicarbonate and protons in blood |
1.0588e+03 (fcn) |
Methodology of Section 2.2.4. The value can be calculated from kinetic data for Carbonic Anhydrase in blood. This data is given in table 2.2 of http://www.chooseclimate.org/benphd/chap2.html
from Sanyal and Maren (1981): Forward Km: 12, backward Km 47, forward kcat 860000, backward kcat 234000. Further the concentration of the enzyme is given as 16.2 +- 0.3 mg/g Hb in (56) which translates to 0.081 mM taking there to be 15 g Hb/dL of blood ((22)) and taking the molecular weight of CA to be about 30 kD (http://www.worthington-biochem.com/CA/default.html). Putting this data together gives
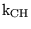 roughly 4975. roughly 4975. |
 |
k_CO2shut |
the rate of transfer of
 across the mitochondrial membrane across the mitochondrial membrane |
9.6141e+01 (fcn) |
|
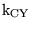 |
k_CY |
rate of combination between
 and the amino groups of haemoglobin and the amino groups of haemoglobin |
3.9763e-02 (fcn) |
methodology of section 2.2 |
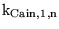 |
k_Cain1n |
normal value of the rate constant for the inflow of calcium into the proximal VSM segment |
1.3863e-05 (fcn) |
see section 4.4 |
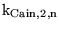 |
k_Cain2n |
normal value of the rate constant for the inflow of calcium into the distal VSM segment |
1.3863e-05 (fcn) |
see section 4.4 |
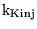 |
k_Kinj |
the rate of injection of potassium into the extracellular space |
0.0000e+00 |
a control parameter used to simulate certain experimental situations, and which must of course normally be set to zero |
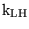 |
k_LH |
the forward rate for the dissociation of lactic acid |
2.3091e+04 (fcn) |
Methodology of Section 2.2.4 |
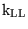 |
k_LL |
rate constant for the diffusion of undissociated lactic acid between the extracellular space and blood |
4.0999e+01 (fcn) |
methodology of section 2.1 |
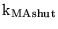 |
k_nMAshut |
rate constant for the malate-aspartate shuttle in the backward direction (NADH reduced in the cytoplasm) |
1.0000e+06 (fcn) |
|
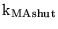 |
k_MAshut |
rate constant for the malate-aspartate shuttle in the forward direction (NADH oxidised in the cytoplasm) |
1.0000e+07 |
|
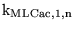 |
k_MLC1acn |
normal rate at which myosin light chains are activated (phosphorylated), say by the action of MLCK in the proximal VSM segment |
1.3863e-01 (fcn) |
methodology of section 2.2 |
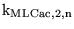 |
k_MLC2acn |
normal rate at which myosin light chains are activated (phosphorylated), say by the action of MLCK in the distal VSM segment |
1.0397e-01 (fcn) |
methodology of section 2.2 |
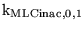 |
k_MLC1inac0 |
the rate of dephosphorylation of myosin in the proximal VSM segment in the absence of NO |
1.1090e-01 (fcn) |
|
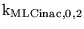 |
k_MLC2inac0 |
the rate of dephosphorylation of myosin in the distal VSM segment in the absence of NO |
1.1090e-01 (fcn) |
|
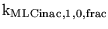 |
k_MLC1inac0_frac |
the rate of dephosphorylation of myosin in the proximal VSM segment in the absence of NO as a fraction of the normal total rate of dephosphorylation |
2.0000e-01 |
(11), p297 states that the total inhibition of NO leads to a 10-60 percent reduction in basal CBF. This is the information we need to use to set this parameter. |
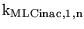 |
k_MLC1inacn |
normal rate of inactivation (dephosphorylation) of myosin heads in the proximal VSM segment |
5.5452e-01 (fcn) |
methodology of section 2.2 |
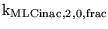 |
k_MLC2inac0_frac |
the rate of dephosphorylation of myosin in the distal VSM segment in the absence of NO as a fraction of the normal total rate of dephosphorylation |
2.0000e-01 |
(11), p297 states that the total inhibition of NO leads to a 10-60 percent reduction in basal CBF. This is the information we need to use to set this parameter. |
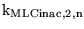 |
k_MLC2inacn |
normal rate of inactivation (dephosphorylation) of myosin heads in the distal VSM segment |
5.8918e-01 (fcn) |
methodology of section 2.2 |
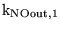 |
k_NOout1 |
the rate of extrusion/degradation of NO from the proximal VSM segment |
6.9315e-03 (fcn) |
methodology of section 2.2 |
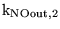 |
k_NOout2 |
the rate of extrusion/degradation of NO from the distal VSM segment |
6.9315e-03 (fcn) |
methodology of section 2.2 |
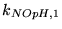 |
k_NOpH_frac1 |
the fraction of normal NO production in the proximal VSM segment which arises as a result of pH |
4.0000e-01 |
important parameter. Evidence for an action of pH via NO is in (57) |
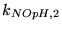 |
k_NOpH_frac2 |
the fraction of normal NO production in the distal VSM segment which arises as a result of pH |
4.0000e-01 |
important parameter. Evidence for an action of pH via NO is in (57) |
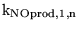 |
k_NOprod1n |
normal rate of production of NO in the muscle cells of the proximal arterial segment |
1.3863e-08 (fcn) |
methodology of section 2.2 |
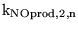 |
k_NOprod2n |
normal rate of production of NO in the muscle cells of the distal arterial segment |
1.3863e-08 (fcn) |
methodology of section 2.2 |
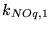 |
k_NOq_frac1 |
the fraction of normal NO production in the proximal VSM segment which arises as a result of shear-stress |
3.0000e-01 |
the implicit assumption is that there is basal NO production beyond that elicited by shear stress and pH - possibly neural. This is backed up by comments on p298 of (11) |
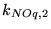 |
k_NOq_frac2 |
the fraction of normal NO production in the distal VSM segment which arises as a result of shear-stress |
3.0000e-01 |
the implicit assumption is that there is basal NO production beyond that elicited by shear stress and pH - possibly neural. This is backed up by comments on p298 of (11) |
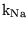 |
k_Na |
the rate of diffusion of sodium ions between blood and extracellular space |
2.0760e+00 (fcn) |
methodology of Section 2.2 |
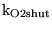 |
k_O2shut |
the rate of diffusion of oxygen between the cell cytoplasm and the mitochondria |
1.7343e+03 (fcn) |
methodology of section 2.1 |
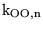 |
k_OOn |
normal rate of diffusion of oxygen between the blood and the extracellular space |
2.4112e+03 (fcn) |
methodology of Section 2.1 - possibly with some corrective factor |
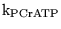 |
k_PCrATP |
the forward rate of reaction for the reaction in which phosphocreatine combines with ADP to give creatine and ATP |
1.6926e+00 (fcn) |
methodology of section 2.2. (22) gives a normal activity in tissue of 0-2.16 microkat (though which direction this is for isn't stated). (58) give Km values of 1.70 mM for MgATP2-, 8.00 mM for Cr, 0.15 mM for MgADP-, and 3.00 mM for PCr. These values translate to about 3-6 for the forward rate constant. |
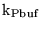 |
k_Pbuf |
The rate at which cellular proteins bind protons |
2.8881e-01 (fcn) |
Methodology of Section 2.2.4 |
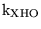 |
k_XHO |
the forward rate constant for the reaction between protonated haemoglobin and oxygen |
1.1743e+05 (fcn) |
methodology of section 2.2.1 |
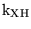 |
k_XH |
the forward rate constant for the reaction between haemoglobin and protons |
1.4733e+06 (fcn) |
methodology of section 2.2.1. This is a caricatured reaction in which one molecule of oxyhaemoglobin combines with
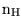 protons protons |
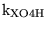 |
k_XO4H |
the forward rate constant for the reaction between oxyhaemoglobin and protons |
9.1166e+02 (fcn) |
see Section 2.2.1 |
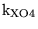 |
k_XO4 |
the forward rate of reaction for the combination of haemoglobin with four molecules of of oxygen |
3.8583e+05 (fcn) |
see Section 2.2.1 |
 |
k_aut1 |
a control parameter normally set to one which can be set to zero to abolish all autoregulation in the proximal VSM segment |
1.0000e+00 |
|
 |
k_aut2 |
a control parameter normally set to one which can be set to zero to abolish all autoregulation in the distal VSM segment |
1.0000e+00 |
|
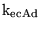 |
k_ecAd |
the rate of diffusion of adenosine between intra- and extracellular spaces |
1.8082e+00 (fcn) |
methodology of section 2.2 |
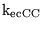 |
k_ecCC |
the rate constant for the diffusion of
 between the extracellular space and the cytoplasm. between the extracellular space and the cytoplasm. |
1.4713e+02 (fcn) |
methodology of section 2.1 |
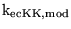 |
k_ecKK_mod |
a parameter used to control the firing rate of the neurons |
1.0000e+00 |
the normal value of this parameter is 1 |
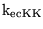 |
k_ecKK |
rate constant for the diffusion of potassium ions between the extracellular space and the cytoplasm. |
1.1006e+00 (fcn) |
see Section 3.1. this parameter embodies the rate of firing of neurons - an increase in this parameter indicates a surge in neural activity... |
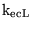 |
k_ecL |
rate constant for the diffusion of undissociated lactic acid between the cytoplasm and the extracellular space |
4.0122e+01 (fcn) |
methodology of section 2.1 |
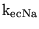 |
k_ecNa |
rate constant for the diffusion of sodium ions between the extracellular space and the cytoplasm |
2.3783e+00 (fcn) |
see Section 3.1. |
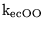 |
k_ecOO |
rate constant for the diffusion of oxygen between the extracellular space and the cytoplasm |
8.7681e+03 (fcn) |
methodology of section 2.1 |
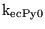 |
k_ecPy0 |
the rate of diffusion of pyruvate ions between intra- and extracellular spaces |
1.0849e+05 (fcn) |
methodology of section 2.2 |
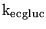 |
k_ecgluc |
rate constant for the diffusion of glucose between the extracellular space and the cytoplasm |
9.8267e+00 (fcn) |
methodology of section 2.1 |
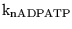 |
k_nADPATP |
the backward rate constant for the conversion of two molecules of ADP to one of ATP and one of AMP |
3.7900e+02 |
Calculated from (5). See Section 4.1 |
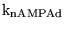 |
k_nAMPAd |
the backwards rate of degradation of AMP to adenosine |
1.3862e-02 (fcn) |
methodology of Section 2.2 |
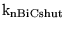 |
k_nBiCshut |
the rate of transfer of bicarbonate ions from mitochondria to cytoplasm |
1.6465e-02 (fcn) |
|
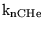 |
k_nCHe |
the backward rate in the conversion of
 to bicarbonate and protons in extracellular space to bicarbonate and protons in extracellular space |
7.4463e+01 (fcn) |
Methodology of Section 2.2.4 |
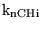 |
k_nCHi |
the backward rate in the conversion of
 to bicarbonate and protons in cytoplasm to bicarbonate and protons in cytoplasm |
2.4752e+02 (fcn) |
Methodology of Section 2.2.4 |
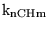 |
k_nCHm |
the backward rate in the conversion of
 to bicarbonate and protons in mitochondria to bicarbonate and protons in mitochondria |
3.4171e+01 (fcn) |
Methodology of Section 2.2.4 |
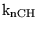 |
k_nCH |
the backward rate in the conversion of
 to bicarbonate and protons in blood to bicarbonate and protons in blood |
1.3403e+06 (fcn) |
Methodology of Section 2.2.4. |
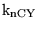 |
k_nCY |
backwards rate in the combination between
 and the amino groups of haemoglobin and the amino groups of haemoglobin |
9.6703e-02 (fcn) |
methodology of section 2.2 |
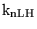 |
k_nLH |
the backward rate for the dissociation of lactic acid |
1.6732e+05 (fcn) |
Methodology of Section 2.2.4 |
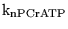 |
k_nPCrATP |
the backward rate of reaction for the reaction in which phosphocreatine combines with ADP to give creatine and ATP |
2.5211e-05 (fcn) |
methodology of section 2.2. (22) gives a normal activity in tissue of 0-2.16 microkat (though which direction this is for isn't stated). (58) give Km values of 1.70 mM for MgATP2-, 8.00 mM for Cr, 0.15 mM for MgADP-, and 3.00 mM for PCr. These values translate to about 3-6e-5 for the backward rate constant. |
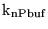 |
k_nPbuf |
The rate at which cellular proteins release protons |
6.7865e+02 (fcn) |
Methodology of Section 2.2.4 |
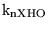 |
k_nXHO |
the backward rate constant for the reaction between protonated haemoglobin and oxygen |
1.3480e+02 (fcn) |
methodology of section 2.2.1 |
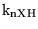 |
k_nXH |
the backward rate constant for the reaction between haemoglobin and protons |
1.9153e+01 (fcn) |
methodology of section 2.2.1. (caricatured) reaction in which one molecule of oxyhaemoglobin combines with
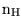 protons protons |
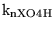 |
k_nXO4H |
the backward rate constant for the reaction between oxyhaemoglobin and protons |
1.8233e-01 (fcn) |
see Section 2.2.1 |
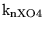 |
k_nXO4 |
backward rate of reaction for the combination of haemoglobin with four molecules of of oxygen |
2.8788e+01 (fcn) |
see Section 2.2.1 |
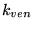 |
k_ven |
a parameter which controls venous compliance |
3.1000e-01 |
0.31 in (14). A larger value means a smaller venous compliance and vice versa |
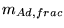 |
m_Ad_frac |
the sensitivity of ATP-sensitive potassium channels to adenosine as a fraction of the total normal response |
2.0000e-01 |
A guess. Edvinsson(11), p314 ff has some experiments on the effects of theophylline (an inhibitor of adenosine) of arterial vessels. This parameter has a significant effect on the duration of hyperemia in the model. |
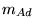 |
m_Ad |
a parameter describing the sensitivity of ATP-sensitive potassium channels to adenosine |
2.0020e-04 (fcn) |
See section 5.3. |
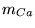 |
m_Ca |
a parameter describing the sensitivity of calicum-sensitive potassium channels to calcium ions |
2.0000e-01 |
The fraction of basal channel activation that is contributed by calcium |
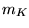 |
m_K |
a parameter describing the sensitivity of inward rectifier potassium channels to exracellular potassium |
5.6395e-03 (fcn) |
See section 5.3 |
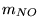 |
m_NO |
a parameter describing the sensitivity of calcium-sensitive potassium channels to NO |
1.0000e-01 |
The fraction of basal channel activation that is contributed by NO |
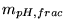 |
m_pH_frac |
the sensitivity of ATP-sensitive potassium channels to extracellular pH as a proportion of the total normal response |
5.0000e-01 |
A guess |
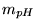 |
m_pH |
a parameter describing the sensitivity of ATP-sensitive potassium channels to extracellular pH |
5.0050e-04 (fcn) |
See section 5.3. |
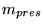 |
m_pres |
a parameter in the relationship between transmural pressure and the conductivity of calcium-sensitive potassium channels |
2.0000e+01 |
A very important parameter for controlling the shape of autoregulation curves. It's units are mmHg. A smaller value indicates a large effect of a change in transmural pressure. A reasonable range is about 10 - 35 mmHg |
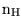 |
n_H |
the number of protons which combine with haemoglobin on average |
5.0000e+00 |
1.22 derived from info on p7 of (22) |
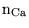 |
n_Ca |
a parameter in the relationship between calcium concentration and the rate of myosin phosphorylation |
2.7000e+00 |
The effect of calcium on myosin phosphorylation is a combination of two effects - calcium binding to calmodulin and Ca/CaM activating MLCK. A hill coefficient of about 2.7 is given in (17) for the binding of calcium to calmodulin. |
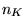 |
n_K |
a parameter describing the sensitivity of inward rectifier potassium channels to exracellular potassium |
2.0000e+00 |
A guess. In order to get a reasonable sensitivity to potassium we need this to be greater than 1. |
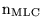 |
n_MLC |
a parameter in the relationship between force generation and myosin phosphorylation in both VSM segments |
1.0000e+00 |
|
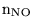 |
n_NO |
a parameter in the relationship between NO concentration and the rate of myosin dephosphorylation |
2.0000e+00 |
In (18) a hill coefficient of 2.1 is given in modelling the production of cGMP as a function of NO concentration |
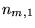 |
n_m1 |
an exponent in the active tension relationship relating the radius of the arteries of the proximal arterial segment to the tension developed |
1.8300e+00 |
1.83 in (14) |
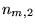 |
n_m2 |
an exponent in the active tension relationship relating the radius of the arteries of the distal arterial segment to the tension developed |
1.7500e+00 |
1.75 in (14) |
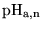 |
pH_an |
normal arterial pH |
7.4100e+00 |
7.41 is a very standard value, e.g. http://faculty.washington.edu/chudler/facts.html |
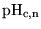 |
pH_bn |
normal pH of capillary blood |
7.3550e+00 (fcn) |
This parameter is there just to make it easier for us to see what is going on with blood pH. Expected blood pH is controlled with expected venous pH. |
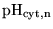 |
pH_tn |
normal cytoplasmic pH |
7.0700e+00 |
7.07 +- 0.03 in (59) for human brains using NMR. 7.0 for immature rat brains in (23). |
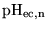 |
pH_en |
normal extracellular pH |
7.2500e+00 |
pH of CSF is given as 7.33 at http://faculty.washington.edu/chudler/facts.html. According to p315-316 of (12), extracellular pH is about 0.3-0.4 units higher than intracellular - i.e. close to that of blood |
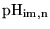 |
pH_mn |
normal mitochondrial pH |
7.6700e+00 (fcn) |
Mitochondrial pH should in general be higher than that in the rest of the cell according to p184 of (12). This is due to the pumping out of electrons during the redox process. |
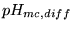 |
pH_mc_diff |
normal pH difference between the cytoplasm and the mitochondria |
6.0000e-01 |
0.6 is the difference given in Cortassa (13) Other authors seem to assume a lower value. |
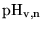 |
pH_vn |
normal venous pH |
7.3000e+00 |
values of about 7.2 are given at http://www.ruhr-uni-bochum.de/anaesthesie/reanima/reani5.htm |
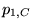 |
p_1_C |
a parameter in the relationship between the rate of oxidative phosphorylation and the membrane and phosphorylation potentials in the Cortassa model |
1.3460e-08 |
value from (13) |
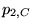 |
p_2_C |
a parameter in the relationship between the rate of oxidative phosphorylation and the membrane and phosphorylation potentials in the Cortassa model |
7.7390e-07 |
value from (13) |
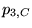 |
p_3_C |
a parameter in the relationship between the rate of oxidative phosphorylation and the membrane and phosphorylation potentials in the Cortassa model |
6.6500e-15 |
value from (13) |
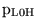 |
p_L0H |
parameter in the expression for the transport of lactate by MCT carriers from extracellular space to blood |
5.0000e-01 |
|
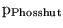 |
p_Phosshut |
a parameter in the equation for the transport of phosphate between cytoplasm and mitochondria |
5.0000e-01 |
Assumed to be accompanied by the transport of a proton. |
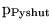 |
p_Pyshut |
a parameter in the equation for the transport of pyruvate between cytoplasm and mitochondria |
5.0000e-01 |
|
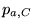 |
p_a_C |
a parameter in the relationship between the rate of oxidative phosphorylation and the membrane and phosphorylation potentials in the Cortassa model |
1.6560e-05 |
value from (13) |
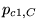 |
p_c1_C |
a parameter in the relationship between the rate of oxidative phosphorylation and the membrane and phosphorylation potentials in the Cortassa model |
9.6510e-14 |
value from (13) |
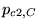 |
p_c2_C |
a parameter in the relationship between the rate of oxidative phosphorylation and the membrane and phosphorylation potentials in the Cortassa model |
4.5850e-14 |
value from (13) |
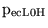 |
p_ecL0H |
parameter in the expression for the transport of lactate by MCT carriers from tissue to extracellular space |
5.0000e-01 |
|
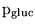 |
p_gluc |
parameter in the expression for the active transport of glucose across the blood-brain barrier |
5.0000e-01 |
0.5 in Figure 2.7 (p47) of (3) |
 |
qn |
normal value of CBF |
1.2500e+01 |
12.5 mL/s (750 mL/min) for whole adult brain at http://faculty.washington.edu/chudler/facts.html. 50 mL/100g brain/min at this site
which translates to about 10.8 mL/s for the whole brain. |
 |
q_csfn |
normal flow rate of CSF |
6.5110e-03 |
|
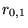 |
r_01 |
a particular radius of the larger arteries |
1.5000e-02 |
0.015 cm in (14). |
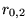 |
r_02 |
a particular radius of the smaller arteries |
7.5000e-03 |
0.0075 cm in (14). |
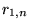 |
r_1n |
normal radius of the arteries in the proximal segment |
2.3501e-02 (fcn) |
|
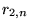 |
r_2n |
normal radius of the arteries in the distal segment |
7.1683e-03 (fcn) |
|
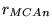 |
r_mcan |
inner radius of the middle cerebral artery in normal state |
1.5000e-01 |
0.15 cm in (14) |
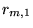 |
r_m1 |
optimal radius at which smooth muscle in the proximal arterial segment exerts maximal force |
2.7000e-02 |
0.027 cm in (14) |
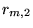 |
r_m2 |
optimal radius at which smooth muscle in the distal arterial segment exerts maximal force |
1.2800e-02 |
0.0128 cm in (14) |
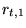 |
r_t1 |
a parameter in the active tension relationship relating the radius of the arteries of the proximal arterial segment to the tension developed |
1.8000e-02 |
0.018 cm in (14) |
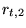 |
r_t2 |
a parameter in the active tension relationship relating the radius of the arteries of the distal arterial segment to the tension developed |
1.7400e-02 |
0.0174 cm in (14) |
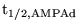 |
k_AMPAd_ht |
halftime for the degradation of AMP to adenosine |
5.0000e+01 |
on p314 of (11) it is stated that the half-life of adenosine in the extracellular space is "less than a minute". |
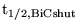 |
k_BiCshut_ht |
The half time for the transport of bicarbonate ions between mitochondria and cytoplasm |
2.2000e+03 |
strangely this seems to have a significant effect on the hyperemia effect (i.e. the extent and duration of overshoot after a period of ischaemia). The existence of a mechanism by which bicarbonate is transported between mitochondria and cytoplasm by an electrogenic process is reported in (60). |
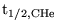 |
k_CHe_ht |
halftime for the production of carbonic acid in extracellular space |
1.1000e+01 |
there is carbonic anhydrase in the extracellular space. CAII (the predominant isoenzyme in brain) values in CSF are given in (61) as 10.2 +- 17.2 micrograms/L in healthy individuals. As the molecular weight is about 30 kD this translates to 3.4e-07 mM, giving
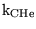 of 0.021. But the uncatalysed rate is about 0.03 per sec. given at http://www.chooseclimate.org/benphd/chap2.html of 0.021. But the uncatalysed rate is about 0.03 per sec. given at http://www.chooseclimate.org/benphd/chap2.html |
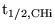 |
k_CHi_ht |
halftime for the production of carbonic acid in cell cytoplasm |
3.2000e+00 |
a guess |
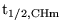 |
k_CHm_ht |
halftime for the production of carbonic acid in mitochondria |
2.5000e+01 |
There is some carbonic anhydrase in mitochondria - (62), but no values are given in this reference... In any case the value for
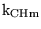 should be larger than 0.03... should be larger than 0.03... |
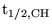 |
k_CH_ht |
halftime for the production and dissociation of carbonic acid |
6.2000e-04 |
chosen to give a good blood pH. Decreasing this parameter increases blood pH but decreases tissue pH by raising blood CO2 levels and hence tissue CO2 levels. |
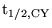 |
k_CY_ht |
halftime for the combination between
 and the amino groups of haemoglobin and the amino groups of haemoglobin |
1.0000e+00 |
a guess |
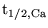 |
k_Ca_ht |
halftime for the inflow of calcium into muscle cells |
5.0000e+00 |
just a guess - a rapid reaction? This is small because the small volume of the muscle compartment is taken into account. The value of this parameter controls the autoregulation time. Making the autoregulation time too slow can cause the model to crash when there are sudden changes in say ABP. |
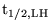 |
k_LH_ht |
halftime for the dissociation of lactic acid |
3.0000e-05 |
a guess - rapid reaction |
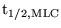 |
k_MLC_ht |
the typical time for a change in calcium/NO to take effect. |
1.0000e+00 |
see the comments on p120-121 of (16) |
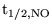 |
k_NO_ht |
normal halftime for the production and loss/degradation of NO |
1.0000e+02 |
100.0 s in abstract of (47) |
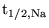 |
k_Na_ht |
halftime for the diffusion of sodium ions between blood and extracellular space |
2.0000e+00 |
a guess |
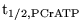 |
k_PCrATP_ht |
halftime for the reaction in which phosphocreatine combines with ADP to give creatine and ATP |
2.0000e-01 |
A halftime of about 0.2 s is compatible with the kinetic data in (58). |
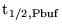 |
k_Pbuf_ht |
Half-time for the reaction in which cellular proteins bind protons |
2.0000e+00 |
|
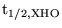 |
k_XHO_ht |
halftime for the combination of protonated haemoglobin with oxygen |
1.0000e-04 |
a guess - fast reaction |
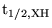 |
k_XH_ht |
halftime for the combination of haemoglobin with protons |
1.0000e-06 |
a guess - fast reaction. |
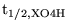 |
k_XO4H_ht |
halftime for the combination of oxyhaemoglobin with protons |
1.0000e-04 |
a guess - fast reaction |
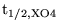 |
k_XO4_ht |
halftime for the combination of haemoglobin with oxygen |
1.0000e-04 |
a guess - fast reaction |
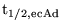 |
k_ecAd_ht |
halftime for the diffusion of adenosine between intra- and extracellular spaces |
6.0000e+01 |
|
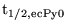 |
k_ecPy0_ht |
halftime for the diffusion of pyruvate ions between intra- and extracellular spaces |
1.0000e-03 |
|
| |
| parameter table ends. |