

Next: Particular calculations: biochemistry
Up: All model parameters
Previous: Estimating rate constants for
Subsections
Metabolic rates and accounting
In this section we work out how the rates of some key reactions are related to each other. This is an example of how parameter setting and the gross model structure are closely related to each other. If we follow the metabolic fates of various chemicals, we naturally get diagrams such as Figure 1.
Figure 1:
The metabolic fates of various substrates in the model.  is the number of protons pumped out of the mitochondrial matrix during the oxidation of two molecules of NADH.
is the number of protons pumped out of the mitochondrial matrix during the oxidation of two molecules of NADH.  is the number of protons pumped out during the oxidation of two molecules of
is the number of protons pumped out during the oxidation of two molecules of
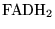 .
. 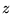 is the number of molecules of ATP produced for each proton that re-enters the mitochondrion.
is the number of molecules of ATP produced for each proton that re-enters the mitochondrion.
![\includegraphics[height = 9cm]{accounting.eps}](img130.png) |
Performing flux balance analysis with the help of such figures gives rise to natural conservation laws of the form ``the normal production of
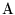 must equal its normal use.'' We note first that the numbers next to arrows need not represent stoichiometries of particular reactions. For example,
must equal its normal use.'' We note first that the numbers next to arrows need not represent stoichiometries of particular reactions. For example, 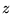 , the total number of ATPs produced per proton reentering the mitochondria, is different from ``the total number of protons which need to flow through the ATPase to phosphorylate one molecule of ADP''.
, the total number of ATPs produced per proton reentering the mitochondria, is different from ``the total number of protons which need to flow through the ATPase to phosphorylate one molecule of ADP''.
In figure 1 and in what follows, for conciseness, we term 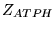 as
as 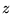 ,
, 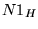 as
as 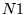 and
and 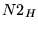 as
as 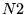 . From the figure we can calculate that:
. From the figure we can calculate that:
We can also indirectly calculate:
- metabolic rate of oxygen: Since one molecule of oxygen is used in the oxidation of every two molecules of
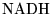 or
or
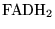 ,
,
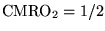 production of
production of
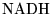 +
+ 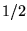 production of
production of
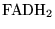 =
=
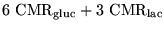 .
.
- rate of production of
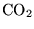 =
=
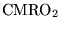 .
.
ATP use, potassium and sodium pumping
We have discussed how the rate of ATP production 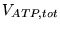 is related to the metabolic rates for glucose and lactate. We now discuss the fate of the ATP produced by the metabolic processes. The basic model assumption is that there are two ATP-consuming processes, ionic homeostasis via
is related to the metabolic rates for glucose and lactate. We now discuss the fate of the ATP produced by the metabolic processes. The basic model assumption is that there are two ATP-consuming processes, ionic homeostasis via
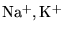 -ATPase, and a lumped process representing all other pathways. The ion pump is given Michaelis Menten dynamics. At steady state let
-ATPase, and a lumped process representing all other pathways. The ion pump is given Michaelis Menten dynamics. At steady state let 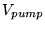 be the rate at which
be the rate at which
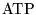 is used to pump potassium back into cells (and sodium back out) i.e.:
is used to pump potassium back into cells (and sodium back out) i.e.:
![\begin{displaymath}
V_{pump} = \ensuremath{\mathrm{\frac{Vmax_{KATP}[ATP_{cyt}]_...
...KATPK}^2 + [K_{ec}^+]_n^2)(Km_{KATPNa}^3 + [Na_{cyt}^+]_n^3)}}}\end{displaymath}](img172.png) |
(51) |
A parameter for which we can find estimates in the literature is the total fraction of ATP used for non-pumping processes, which we term 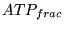 . We then have the relationship:
. We then have the relationship:
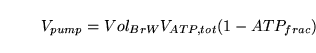 |
(52) |
The value of 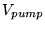 obtained from this equation can now be used in equation 51 to calculate
obtained from this equation can now be used in equation 51 to calculate
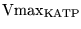 . Further we have that:
. Further we have that:
![\begin{displaymath}
\ensuremath{\mathrm{k_{ecKK}([K_{cyt}^+]_{n} - [K_{ec}^+]_{n...
...{\mathrm{k_{ecNa}([Na^+_{ec}]_n - [Na^+_{cyt}]_n)}}= 3V_{pump}
\end{displaymath}](img176.png) |
(53) |
which can be used to calculate
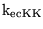 and
and
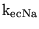 .
.
Finally, we have for the rate of
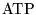 use for non-pumping purposes:
use for non-pumping purposes:
![\begin{displaymath}
\frac{\ensuremath{\mathrm{Vmax_{ATPuse}}}\ensuremath{\mathrm...
...cyt}]_n}}} = \frac{Vol_{BrW}}{Vol_{cyt}}V_{ATP, tot}ATP_{frac}
\end{displaymath}](img179.png) |
(54) |
which can be used to calculate
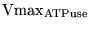 .
.


Next: Particular calculations: biochemistry
Up: All model parameters
Previous: Estimating rate constants for
Murad Banaji
2004-07-08
![\includegraphics[height = 9cm]{accounting.eps}](img130.png)
![\includegraphics[height = 9cm]{accounting.eps}](img130.png)
![]() as
as ![]() ,
, ![]() as
as ![]() and
and ![]() as
as ![]() . From the figure we can calculate that:
. From the figure we can calculate that:
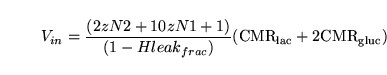
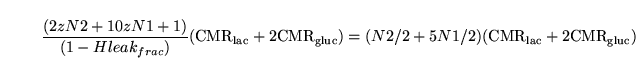
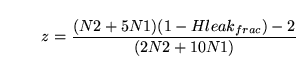
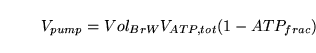
![\begin{displaymath}
\ensuremath{\mathrm{k_{ecKK}([K_{cyt}^+]_{n} - [K_{ec}^+]_{n...
...{\mathrm{k_{ecNa}([Na^+_{ec}]_n - [Na^+_{cyt}]_n)}}= 3V_{pump}
\end{displaymath}](img176.png)
![]() use for non-pumping purposes:
use for non-pumping purposes:
![\begin{displaymath}
\frac{\ensuremath{\mathrm{Vmax_{ATPuse}}}\ensuremath{\mathrm...
...cyt}]_n}}} = \frac{Vol_{BrW}}{Vol_{cyt}}V_{ATP, tot}ATP_{frac}
\end{displaymath}](img179.png)