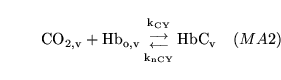
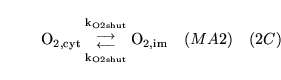
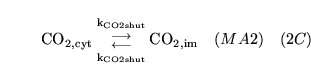
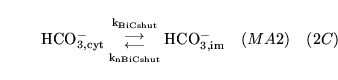
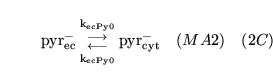
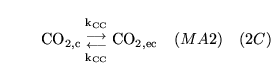
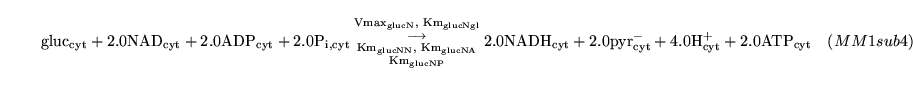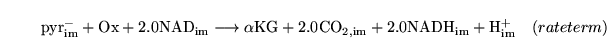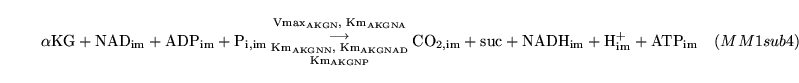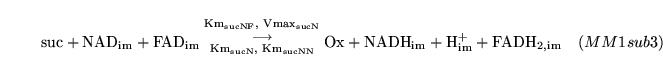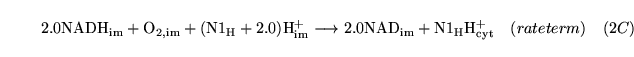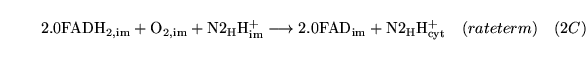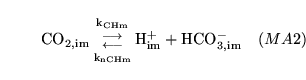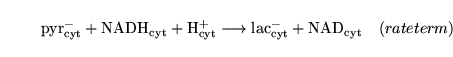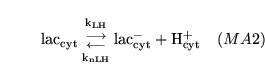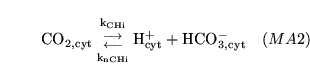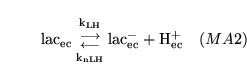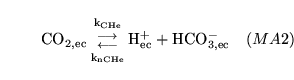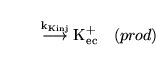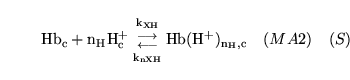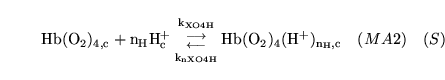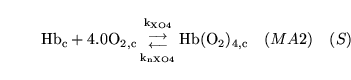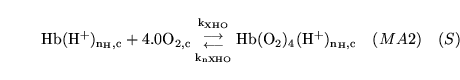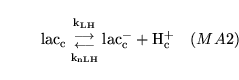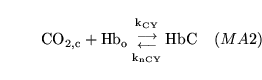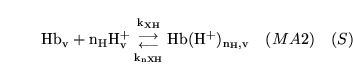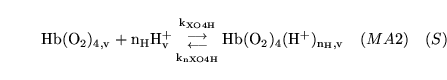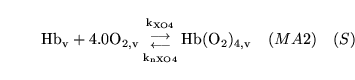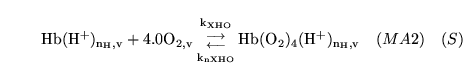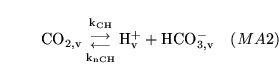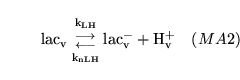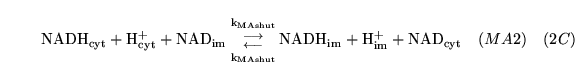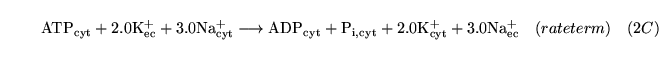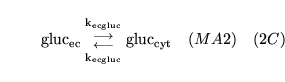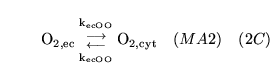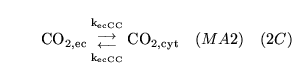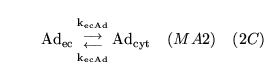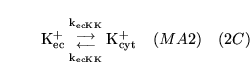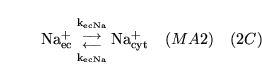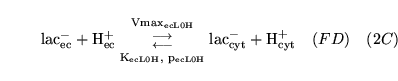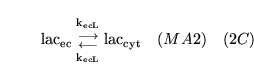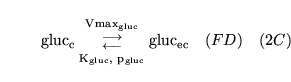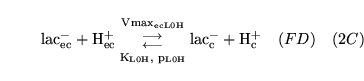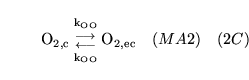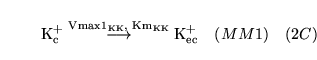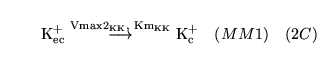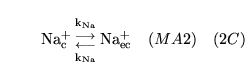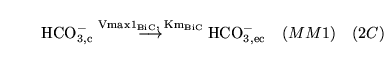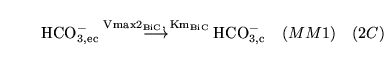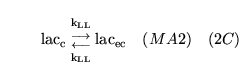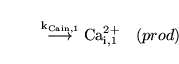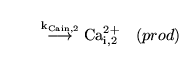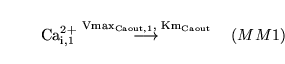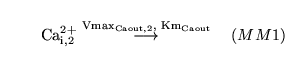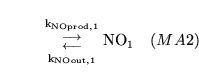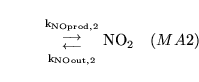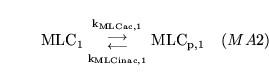The following substances are transported convectively from the capillaries to the veins:
 ,
,
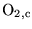 ,
,
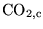 ,
,
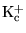 ,
,
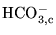 ,
,
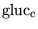 ,
,
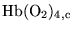 ,
,
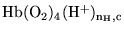 ,
,
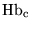 ,
,
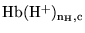 ,
,
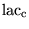 ,
,
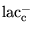 ,
,
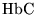 ,
,
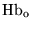 ,
,
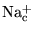 .
.
The following substances are transported convectively out of the extracellular space into the venous system (via CSF drainage):
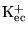 ,
,
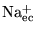 ,
,
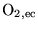 ,
,
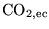 ,
,
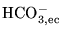 ,
,
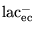 ,
,
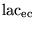 .
.
| brief name (process type: site) |
process name |
description |
other comments |
| |
|
|
|
glycol (reaction: tissue cell cytoplasm) |
glycolysis in one step |
a one way Michaelis Menten reaction which is assumed to capture the process of glycolysis |
Glycolysis is actually 10 reactions catalysed by hexokinase, phosphoglucoisomerase, phosphofructokinase, fructose biphosphate aldolase, triose phosphate isomerase, glyceraldehyde-3-P dehydrogenase, phosphoglycerate kinase, phosphoglycerate mutase, enolase and pyruvate kinase. Useful web-based outlines of glycolysis can be found here
and here |
Krebs1 (reaction: mitochondria) |
PDH reaction plus first stages of the TCA cycle |
A lumped reaction consisting of the pyruvate dehydrogenase reaction, and the first three stages of the TCA cycle. |
This is a combination of four reactions catalysed by pyruvate dehydrogenase, citrate synthase, aconitase and isocitrate dehydrogenase. The first of these is the bridging reaction between glycolysis and the TCA cycle. A useful web-based outline of the cycle is at http://www.gwu.edu/~mpb/citric.htm |
Krebs2 (reaction: mitochondria) |
middle stages of the TCA cycle |
A lumped reaction consisting of two stages of the TCA cycle |
This is actually a combination of two reactions catalysed by alpha-ketoglutarate dehydrogenase and succinyl-CoA synthase. A useful web-based outline of the cycle is at http://www.gwu.edu/~mpb/citric.htm |
Krebs3 (reaction: mitochondria) |
final stages of the TCA cycle |
A lumped reaction consisting of the final three stages of the TCA cycle |
This is actually an amalgamation of three reactions catalysed by succinate dehydrogenase, fumarase and malate dehydrogenase. A useful web-based outline of the cycle is at http://www.gwu.edu/~mpb/citric.htm |
Noxy (reaction: mitochondria (with protons pumped to the cytoplasm)) |
oxidation of NADH |
A caricatured reaction summarising the oxidation of NADH in the electron transport chain. |
oxygen is used up and protons are pumped out of the mitochondria. A more detailed description of the electron transport chain might follow Korzeniewski (2) |
Foxy (reaction: mitochondria (with protons pumped to the cytoplasm)) |
oxidation of
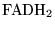 |
A caricatured reaction summarising the oxidation of
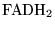 in the electron transport chain. in the electron transport chain. |
oxygen is used up and protons are pumped out of the mitochondria. The models of Korzeniewski (2) don't treat FAD at all. |
Hyleak (reaction: between cell cytoplasm and mitochondria) |
proton leak |
The proton leak through the mitochondrial membrane |
We assume an exponential relationship of the rate of leak on the proton motive force, following for example (3) and (2) |
Cortoxphos (reaction: mitochondria (protons enter from cytoplasm)) |
oxidative phosphorylation |
A reaction summarising oxidative phosphorylation with the functional form for the rate term taken from the Cortassa et al model (4) |
|
CO2toHm (reaction: ) |
mitochondrial production and dissociation of carbonic acid |
The production and dissociation of carbonic acid in mitochondria |
See comments for CO2toH1 |
PytoL (reaction: tissue cell cytoplasm) |
lactate to pyruvate interconversion |
A two way Michaelis Menten reaction in which lactate is converted to pyruvate along with the reduction of NAD. |
The rate term and the constants are taken from (5) |
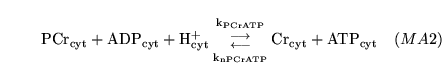
PCrtoATP (reaction: tissue cell cytoplasm) |
conversion of PCr to ATP |
A two way mass action reaction representing the interconversion of PCr + ADP to Cr + ATP. |
the reaction is catalysed by creatine kinase, but we assume that it is fast and hence normally near equilibrium, and hence can be treated as a mass action reaction |
ATPuse (reaction: ) |
non-pumping ATP use |
A single process representing all usage of ATP other than that involved in neural membrane potential maintenance |
In treating this as a single caricatured process we follow the example of (6). |
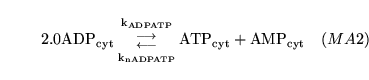
aden1 (reaction: tissue cell cytoplasm) |
adenlyate kinase reaction |
a two way reaction in which two molecules of ADP are converted into one of ATP and one of AMP. |
In reality the enzyme catalysing the reaction is adenylate kinase. However we assume that the reaction is always near equilibrium, and hence can be given mass action dynamics. In this we follow the literature, e.g. (7). |
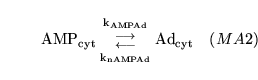
aden2 (reaction: tissue cell cytoplasm) |
AMP degradation |
a two-way reaction in which AMP is interconverted with adenosine |
Technically this reaction should involve phosphate too. But the total quantity of adenosine is tiny, and it participates in no further reactions, so we ignore the phosphate production. Various nucleotidases catalyse this reaction (8) which in inhibited by ATP and ADP. Because they mostly have a high Km for AMP we treat the process as a mass-action reaction. |
lactic (reaction: tissue cell cytoplasm) |
lactic acid dissociation in cytoplasm |
The dissociation of lactic acid to give lactate ions and protons |
|
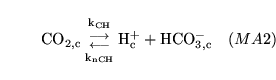
CO2toH1 (reaction: ) |
cytoplasmic production and dissociation of carbonic acid |
The production and dissociation of carbonic acid in cell cytoplasm |
The process is actually an amalgamation of the process of carbonic acid production, catalysed by carbonic anhydrase, and the dissociation of the acid. It is quite standard to treat this as one process - see for example p9 of (9). |
genbuf (reaction: ) |
Calcium production in the proximal VSM segment |
A general buffer reaction in representing the action of cell proteinssite: cell cytoplasmothercoms: The details of the concentration. We assume that cellular proteins can perform a buffer function to a similar extent to the
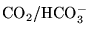 buffer system. ******Caentry1A zeroth order process in which calcium is "produced" - i.e. enters the cells - in the proximal VSM segment buffer system. ******Caentry1A zeroth order process in which calcium is "produced" - i.e. enters the cells - in the proximal VSM segment |
the rate constant for this entry depends on the conductivity of the calcium channels and on the membrane potential |
elactic (reaction: extracellular space ) |
lactic acid dissociation in extracellular space |
The dissociation of lactic acid to give lactate ions and protons |
|
CO2toHe (reaction: ) |
extracellular production and dissociation of carbonic acid |
The production and dissociation of carbonic acid in extracellular space |
See comments for CO2toH1 |
potinject (reaction) |
|
|
|
hemacid1 (reaction: ) |
protonation of deoxygenated haemoglobin |
The combination of de-oxygenated haemoglobin with 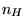 protons protons |
This is actually a caricature of a large number of reactions involving different numbers of protons binding to haemoglobin |
hemacid2 (reaction: ) |
protonation of oxygenated haemoglobin |
The combination of oxygenated haemoglobin with 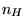 protons protons |
This is actually a caricature of a large number of reactions involving different numbers of protons binding to oxy-haemoglobin |
hemoxy1 (reaction: ) |
oxygenation of unprotonated haemoglobin |
The combination of unprotonated haemoglobin oxygen |
This is actually a caricature of a four stage reaction in which oxygen molecules sequentially bind to haemoglobin. Because of co-operativity, a Hill coefficient of 2.5 is given to oxygen in the reaction |
hemoxy2 (reaction: ) |
oxygenation of protonated haemoglobin |
The combination of protonated haemoglobin oxygen |
This is actually a caricature of a four stage reaction in which oxygen molecules sequentially bind to haemoglobin. Because of co-operativity, a Hill coefficient of 2.5 is given to oxygen in the reaction |
CO2toH (reaction: ) |
capillary production and dissociation of carbonic acid |
The production and dissociation of carbonic acid in capillary blood |
See comments for CO2toH1 |
lacticb (reaction: capillary blood ) |
lactic acid dissociation in capillary blood |
The dissociation of lactic acid to give lactate ions and protons |
|
carbam (reaction: ) |
combination of
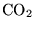 with haemoglobin in capillaries with haemoglobin in capillaries |
combination of
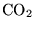 with binding sites (terminal amino groups) on haemoglobin in capillaries with binding sites (terminal amino groups) on haemoglobin in capillaries |
We assume that this combination is independent of the oxygenation and protonation of haemoglobin. See this website
for a description of the process. |
venhemacid1 (reaction: ) |
protonation of deoxygenated haemoglobin in venous blood |
The combination of de-oxygenated haemoglobin with 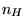 protons in venous blood protons in venous blood |
This is actually a caricature of a large number of reactions involving different numbers of protons binding to haemoglobin |
venhemacid2 (reaction: ) |
protonation of oxygenated haemoglobin in venous blood |
The combination of oxygenated haemoglobin with 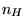 protons in venous blood protons in venous blood |
This is actually a caricature of a large number of reactions involving different numbers of protons binding to oxy-haemoglobin |
venhemoxy1 (reaction: ) |
oxygenation of unprotonated haemoglobin in venous blood |
The combination of unprotonated haemoglobin oxygen in venous blood |
This is actually a caricature of a four stage reaction in which oxygen molecules sequentially bind to haemoglobin. Because of co-operativity, a Hill coefficient of 2.5 is given to oxygen in the reaction |
venhemoxy2 (reaction: ) |
oxygenation of protonated haemoglobin in venous blood |
The combination of protonated haemoglobin oxygen in venous blood |
This is actually a caricature of a four stage reaction in which oxygen molecules sequentially bind to haemoglobin. Because of co-operativity, a Hill coefficient of 2.5 is given to oxygen in the reaction |
venCO2toH (reaction: ) |
venous production and dissociation of carbonic acid |
The production and dissociation of carbonic acid in venous blood |
See comments for CO2toH1 |
venlacticb (reaction: venous blood) |
lactic acid dissociation in venous blood |
The dissociation of lactic acid to give lactate ions and protons |
|
vencarbam (reaction: ) |
combination of
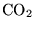 with haemoglobin in veins with haemoglobin in veins |
combination of
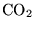 with binding sites (terminal amino groups) on haemoglobin in venous blood with binding sites (terminal amino groups) on haemoglobin in venous blood |
see comments for carbam |
MAshuttle (reaction) |
|
|
|
pyrshuttle (reaction: between cytoplasm and mitochondria) |
pyruvate transport |
The facilitated diffusion of pyruvate between cytoplasm and mitochondria coupled to the transport of a proton |
Like the transport of lactate this is assumed to occur primarily via monocarboxylate transporters |
Phosshuttle (reaction: between cytoplasm and mitochondria) |
Phosphate transport |
The facilitated diffusion process in which phosphate ions are transported between cytoplasm and mitochondria. Transport is coupled to the transport of a proton |
Although the transport is actually coupled to the antiport of a hydroxide ion rather than the symport of a proton - (10) - we assume that practically this makes little difference. Korzeniewski (2) has a non-saturating simple diffusion term for this process. |
ATPshuttle1 (reaction: mitochondrial membrane) |
ATP-ADP antiport |
A reaction representing the action of ANT transporters which exchange ATP and ADP across the mitochondrial membrane |
The functional form is taken from Cortassa (4). |
O2shuttle (reaction: between cytoplasm and mitochondria) |
diffusion of oxygen |
The two way diffusion of oxygen between cytoplasm and mitochondria |
|
CO2shuttle (reaction: between cytoplasm and mitochondria) |
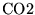 transport transport |
A two way diffusive process representing movement of
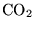 between cytoplasm and mitochondria between cytoplasm and mitochondria |
|
BiCshuttle (reaction: between cytoplasm and mitochondria) |
bicarbonate transport |
A two way mass action process representing movement of bicarbonate between cytoplasm and mitochondria |
The two rate constants are allowed to differ, implying implicitly that the process might be active/linked to other transport processes. We are not certain of the importance of this process in the maintenance of mitochondrial pH. |
potpump (reaction: ) |
active sodium potassium exchange |
The active pumping of potassium into cells and sodium out of cells by
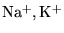 -ATPase. Three sodium ions are pumped out for every two potassium ions pumped in. -ATPase. Three sodium ions are pumped out for every two potassium ions pumped in. |
See (11) for some Km values |
ECdiffgluc (reaction: between extracellular space and cell cytoplasm) |
glucose transport from extracellular space to cells |
Diffusion of glucose from extracellular space to cell cytoplasm |
|
ECdiffO (reaction: between extracellular space and cell cytoplasm) |
diffusion of
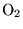 out of cells out of cells |
Two way diffusion of
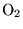 between cell cytoplasm and extracellular space between cell cytoplasm and extracellular space |
|
ECdiffCO2 (reaction: extracellular space and cell cytoplasm) |
diffusion of
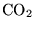 out of cells out of cells |
Two way diffusion of
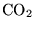 between cell cytoplasm and extracellular space between cell cytoplasm and extracellular space |
|
ECdiffPy0 (reaction: extracellular space and cell cytoplasm) |
diffusion of pyruvate ions out of cells |
Two way diffusion of pyruvate ions between cell cytoplasm and extracellular space |
|
ECdiffAd (reaction: extracellular space and cell cytoplasm) |
diffusion of adenosine out of cells |
Two way diffusion of adenosine between cell cytoplasm and extracellular space |
This process is important because it is adenosine outside cells has a dilatory effect on vascular smooth muscle ((12), (13)). Extracellular adenosine either exits cells via bi-directional nucleoside transporters or is produced by the extracellular degradation of AMP ((8)). We currently only treat the first of these processes. Adenosine is actually transported across the BBB by nucleoside transporters (see p121 of (14)) with a Km much greater than its concentration. |
ECdiffK (reaction: ) |
potassium diffusion between cells and extracellular space |
The background transport of potassium ions between cytoplasm and extracellular space |
actually this process represents the net outcome of many neural firing processes. Representing it as a diffusive process is very simplistic, but avoids modelling neural electrophysiology. The parameters for this and sodium transport can be altered to simulate increased rates of neural firing. |
ECdiffNa (reaction: ) |
sodium diffusion between cells and extracellular space |
The background transport of sodium ions between cytoplasm and extracellular space |
actually this process, like that for potassium, represents the net outcome of many neural firing processes. It is linked to the potassium process and the two could perhaps be treated as a single process to ensure the right stoichiometry. |
ECdiffL0H (reaction: ) |
lactate transport between extracellular space and cells |
Facilitated diffusion of lactate ions co-transported with a proton, between extracellular space and cell cytoplasm |
Represents the action of monocarboxylate transporters (MCTs) - see for example chapter 7 of (14) |
ECdiffL (reaction: ) |
lactic acid diffusion between extracellular space and cells |
Passive diffusion of undissociated lactic acid between extracellular space and cell cytoplasm |
we assume that normally this represents a small proportion of transport, but could become significant during lactic acidosis |
BEdiffgluc (reaction: between blood and extracellular space) |
glucose transport from capillary blood to extracellular space |
Facilitated diffusion of glucose between capillary blood and extracellular space. |
|
BEdiffL0H (reaction: between blood and extracellular space) |
lactate transport between extracellular space and blood |
Facilitated diffusion of lactate ions co-transported with a proton, between extracellular space and capillary blood |
Represents the action of monocarboxylate transporters (MCTs) - see for example chapter 7 of (14) |
BEdiffO (reaction: between blood and extracellular space) |
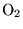 transport between blood and extracellular space transport between blood and extracellular space |
Two way diffusion of
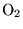 between blood and extracellular space between blood and extracellular space |
|
BEdiffCO2 (reaction: between blood and extracellular space) |
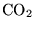 transport between blood and extracellular space transport between blood and extracellular space |
Two way diffusion of
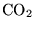 between blood and extracellular space between blood and extracellular space |
|
BEdiffK1 (reaction: between blood and extracellular space) |
potassium transport from blood to extracellular space |
A one way Michaelis Menten reaction in which potassium ions are transported from blood to extracellular space. It is one part of a two way process. |
|
BEdiffK2 (reaction: between blood and extracellular space) |
potassium transport from extracellular space to blood |
A one way Michaelis Menten reaction in which potassium ions are transported from extracellular space to blood. It is one part of a two way process. |
|
BEdiffNa (reaction: between blood and extracellular space) |
sodium diffusion between blood and extracellular space |
The diffusion of sodium ions between blood and extracellular space |
This process is assumed to be diffusive, because the concentrations of sodium ions in the two compartments are much the same |
BEdiffBiC1 (reaction: between blood and extracellular space) |
bicarbonate transport from blood to extracellular space |
A one way Michaelis Menten reaction in which bicarbonate ions are transported from blood to extracellular space. It is one part of a two way process. |
|
BEdiffBiC2 (reaction: between blood and extracellular space) |
bicarbonate transport from extracellular space to blood |
A one way Michaelis Menten reaction in which bicarbonate ions are transported from extracellular space to blood. It is one part of a two way process. |
|
BEdiffL (reaction: between blood and extracellular space) |
lactic acid diffusion between extracellular space and blood |
Passive diffusion of undissociated lactic acid between extracellular space and capillary blood |
we assume that normally this represents a small proportion of transport, but could become significant during lactic acidosis |
Caentry1 (reaction: ) |
Calcium production in the proximal VSM segment |
A zeroth order process in which calcium is "produced" - i.e. enters the cells - in the proximal VSM segment |
the rate constant for this entry depends on the conductivity of the calcium channels and on the membrane potential |
Caentry2 (reaction: ) |
Calcium production in the distal VSM segment |
A zeroth order process in which calcium is "produced" - i.e. enters the cells - in the distal VSM segment |
the rate constant for this entry depends on the conductivity of the calcium channels and on the membrane potential |
Caexit1 (reaction: proximal VSM segment) |
VSM expulsion in the proximal VSM segment |
A one way Michaelis Menten process in which calcium is expelled from the cell cytoplasm in the proximal VSM segment |
This is known to be an active process, and hence presumably can saturate |
Caexit2 (reaction: distal VSM segment) |
VSM expulsion in the distal VSM segment |
A one way Michaelis Menten process in which calcium is expelled from the cell cytoplasm in the distal VSM segment |
This is known to be an active process, and hence presumably can saturate |
NObuf1 (reaction: proximal VSM segment) |
NO dynamics in the proximal VSM segment |
A reaction comprising the zeroth order production of NO and the first order degradation of NO in the proximal VSM segment |
What is captured in this processes - extracellular production of NO (mainly endothelial), diffusion into the VSM cells, subsequent diffusion out of the cells and reaction with
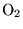 - see (15). - see (15). |
NObuf2 (reaction: distal VSM segment) |
NO dynamics in the proximal VSM segment |
A reaction comprising the zeroth order production of NO and the first order degradation of NO in the distal VSM segment |
see comment for NObuf1 |
MLC1inac (reaction: proximal VSM segment) |
phosphorylation of MLC in the proximal VSM segment |
A two way mass action reaction in which myosin light chains are phosphorylated (activated) and dephosphorylated (inactivated) in the the proximal arterial VSM segment |
see for example (14), (16), (17) for overviews. Regulation occurs because the two rates are affected by calcium and NO concentrations |
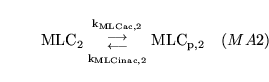
MLC2inac (reaction: distal VSM segment) |
phosphorylation of MLC in the distal VSM segment |
A two way mass action reaction in which myosin light chains are phosphorylated (activated) and dephosphorylated (inactivated) in the the distal arterial VSM segment |
see for example (14), (16), (17) for overviews. Regulation occurs because the two rates are affected by calcium and NO concentrations |
qrel (algebraic relation: ) |
cerebral blood flow |
Equation determining cerebral blood flow from the Ursino model in (1). |
athough it is determined in the distal arterial segment, it should be much the same everywhere, assuming that CSF production and outflow are minor processes from this point of view |
P_crel (algebraic relation: ) |
Capillary pressure |
Equation determining the capillary pressure from the Ursino model in (1). |
This equation is actually simply a conservation law for the fluid flowing in and out of the capillary compartment where the compartment is assumed to have no compliance |
sigma_v1rel (algebraic relation: ) |
viscous stress in the proximal arterial walls |
Equation determining viscous stress in the vessel walls of the proximal arterial segment, from the Ursino model in (1). |
assumed to be proportional to the rate of change of vessel radius |
sigma_v2rel (algebraic relation: ) |
viscous stress in the distal arterial walls |
Equation determining viscous stress in the vessel walls of the distal arterial segment, from the Ursino model in (1). |
assumed to be proportional to the rate of change of vessel radius |
P_1rel (algebraic relation: proximal arterial segment) |
pressure in the proximal arterial segment |
Equation determining pressure in the proximal arterial segment from the Ursino model in (1) |
determined by the balance of elastic, active and viscous forces across the vessel wall |
P_2rel (algebraic relation: distal arterial segment) |
pressure in the distal arterial segment |
Equation determining pressure in the distal arterial segment from the Ursino model in (1) |
determined by the balance of elastic, active and viscous forces across the vessel wall |
sigma_e1rel (algebraic relation: vessel walls of the proximal arterial segment) |
elastic stress in the vessel walls in the proximal arterial segment |
Equation determining the elastic stress in the vessel walls in the proximal arterial segment from the Ursino model in (1) |
a function of vessel radius. Can be positive or negative. |
sigma_e2rel (algebraic relation: vessel walls of the distal arterial segment) |
elastic stress in the vessel walls in the distal arterial segment |
Equation determining the elastic stress in the vessel walls in the distal arterial segment from the Ursino model in (1) |
a function of vessel radius. Can be positive or negative. |
T_e1rel (algebraic relation: vessel walls of the proximal arterial segment) |
elastic tension in the vessel walls in the proximal arterial segment |
Elastic tension in the vessel walls in the proximal arterial segment from the Ursino model in (1) |
proportional to elastic stress and vessel wall thickness |
T_e2rel (algebraic relation: vessel walls of the distal arterial segment) |
elastic tension in the vessel walls in the distal arterial segment |
Elastic tension in the vessel walls in the distal arterial segment from the Ursino model in (1) |
proportional to elastic stress and vessel wall thickness |
T_m1rel (algebraic relation: vessel walls of the proximal arterial segment) |
Active tension in the vessel walls in the proximal arterial segment |
Active (muscular) tension in the vessel walls in the proximal arterial segment from the Ursino model in (1) |
A key bridging variable between the wider model and the Ursino model. This tension depends on vessel radius, but also on T_max1 - the maximum active muscle tension, which is itself determined by the dynamics of vascular smooth muscle |
T_m2rel (algebraic relation: vessel walls of the distal arterial segment) |
Active tension in the vessel walls in the distal arterial segment |
Active (muscular) tension in the vessel walls in the distal arterial segment from the Ursino model in (1) |
see comment for T_m1rel |
T_v1rel (algebraic relation: vessel walls of the proximal arterial segment) |
viscous tension in the vessel walls in the proximal arterial segment |
Equation determining the viscous tension in the vessel walls in the proximal arterial segment from the Ursino model in (1) |
proportional to viscous stress and vessel wall thickness |
T_v2rel (algebraic relation: vessel walls of the distal arterial segment) |
viscous tension in the vessel walls in the distal arterial segment |
Equation determining the viscous tension in the vessel walls in the distal arterial segment from the Ursino model in (1) |
proportional to viscous stress and vessel wall thickness |
h_1rel (algebraic relation: vessel walls of the proximal arterial segment) |
thickness of vessel walls in the proximal arterial segment |
Equation determining the thickness of vessel walls in the proximal arterial segment from the Ursino model in (1) |
determined by assuming the conservation of wall volume |
h_2rel (algebraic relation: vessel walls of the distal arterial segment) |
thickness of vessel walls in the distal arterial segment |
Equation determining the thickness of vessel walls in the distal arterial segment from the Ursino model in (1) |
determined by assuming the conservation of wall volume |
G_1rel (algebraic relation: ) |
conductivity of the proximal arterial segment |
Equation determining the conductivity of the proximal arterial segment as a function of vessel radius, from the Ursino model in (1). |
proportional to the fourth power of the radius |
G_2rel (algebraic relation: ) |
conductivity of the distal arterial segment |
Equation determining the conductivity of the distal arterial segment as a function of vessel radius, from the Ursino model in (1). |
proportional to the fourth power of the radius |
V_1rel (algebraic relation: ) |
Volume of the proximal arterial segment |
Equation determining the volume of the proximal arterial segment as a function of vessel radius, from the Ursino model in (1). |
proportional to the square of the radius |
V_2rel (algebraic relation: ) |
Volume of the distal arterial segment |
Equation determining the volume of the distal arterial segment as a function of vessel radius, from the Ursino model in (1). |
proportional to the square of the radius |
C_virel (algebraic relation: ) |
venous compliance |
Equation determining the compliance of the veins from the Ursino model in (1). |
Assumed to vary inversely with transmural pressure |
G_vsrel (algebraic relation: ) |
conductance of the terminal veins |
Equation determining the conductance of the terminal veins from the Ursino model in (1). |
The assumption is that the system behaves as a starling resistor |
r_mcarel (algebraic relation: ) |
MCA radius |
An estimate of the radius of the middle cerebral artery |
a function of the logarithm of the transmural pressure |
V_mcarel (algebraic relation: ) |
MCA volume |
An estimate of the volume of the middle cerebral artery |
proportional to the square of the MCA radius |
T_max1nrel (algebraic relation: ) |
maximum muscle tension in the proximal arterial segment |
The way that T_max1 - the maximum active muscle tension in the proximal arterial segment - depends on the level of muscle activation. |
A control parameter 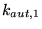 is included so that we can destroy all autoregulation in this segment if so desired is included so that we can destroy all autoregulation in this segment if so desired |
T_max2nrel (algebraic relation: ) |
maximum muscle tension in the distal arterial segment |
The way that T_max2 - the maximum active muscle tension in the distal arterial segment - depends on the level of muscle activation. |
A control parameter 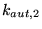 is included so that we can destroy all autoregulation in this segement if so desired is included so that we can destroy all autoregulation in this segement if so desired |
M_1nrel (algebraic relation: ) |
level of muscle activation in proximal VSM segment |
The way that M_1 - the level of muscle activation in the proximal VSM segment - depends on levels of phosphorylated myosin |
see (18) for detailed modelling work, or (19) for a more pragmatic approach |
M_2nrel (algebraic relation: ) |
level of muscle activation in distal VSM segment |
The way that M_2 - the level of muscle activation in the distal VSM segment - depends on levels of phosphorylated myosin |
see comments for M_1nrel |
Vmax_glucNrel (algebraic relation: tissue cell cytoplasm) |
Vmax for glycolysis |
a relation describing how the Vmax for glycolysis depends on the cytoplasmic concentrations of of ATP and AMP |
This is the standard form for allosteric activation, i.e.
![$\ensuremath{\mathrm{Vmax \to Vmax/(1 + K_a/[A])}}$](img117.png) . We have added the term in the numerator, so that the normal Vmax is . We have added the term in the numerator, so that the normal Vmax is
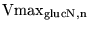 . . |
PMFrel (algebraic relation: across the mitochondrial membrane) |
proton motive force |
The way in which the proton motive force moving protons across the mitochondrial membrane depends on the membrane potential and the pH gradient |
|
PD_mitrel (algebraic relation: mitochondrial membrane) |
mitochondrial membrane potential |
The Korzeniewski assumption that membrane potential is proportional to pH difference |
(20), (2) and the references therein. |
gKpot1rel (algebraic relation: VSM membrane in proximal segment) |
IR potassium channel conductivity in the proximal VSM segment |
How the conductivity of inward rectifier potassium channels in the proximal VSM segment depends on the extracellular potassium concentration |
see for example (21) |
gKpot2rel (algebraic relation: VSM membrane in distal segment) |
IR potassium channel conductivity in the distal VSM segment |
How the conductivity of inward rectifier potassium channels in the distal VSM segment depends on the extracellular potassium concentration |
see for example (21) |
gKV1rel (algebraic relation: VSM membrane in proximal segment) |
voltage gated potassium channel conductivity in the proximal VSM segment |
How the conductivity of voltage gated potassium channels in the proximal VSM segment depends on membrane potential |
There is both activation and inactivation. Functional form taken from see (22). |
gKV2rel (algebraic relation: VSM membrane in distal segment) |
voltage gated potassium channel conductivity in the distal VSM segment |
How the conductivity of voltage gated potassium channels in the distal VSM segment depends on membrane potential |
There is both activation and inactivation. Functional form taken from see (22). |
gKATP1rel (algebraic relation: VSM membrane in proximal segment) |
ATP-sensitive potassium channel conductivity in the proximal VSM segment |
How the conductivity of ATP-sensitive potassium channels in the proximal VSM segment depends on pH and adenosine concentrations in the extracellular space |
see (23) for pH, (12), (13) for adenosine. One further stimulus to which these channels respond is intracellular ATP itself (24), but we currently ignore this |
gKATP2rel (algebraic relation: VSM membrane in distal segment) |
ATP-sensitive potassium channel conductivity in the distal VSM segment |
How the conductivity of ATP-sensitive potassium channels in the distal VSM segment depends on pH and adenosine concentrations in the extracellular space |
see (23) for pH, (12), (13) for adenosine. One further stimulus to which these channels respond is intracellular ATP itself (24), but we currently ignore this |
gKpres1rel (algebraic relation: VSM membrane in proximal segment) |
calcium-sensitive potassium channel conductivity in the proximal VSM segment |
How the conductivity of calcium-sensitive potassium channels in the proximal VSM segment depends on NO, calcium, membrane potential, and transmural pressure |
For pressure, see (25), (26). For NO see (27) |
gKpres2rel (algebraic relation: VSM membrane in distal segment) |
calcium-sensitive potassium channel conductivity in the distal VSM segment |
How the conductivity of calcium-sensitive potassium channels in the distal VSM segment depends on NO, calcium, membrane potential, and transmural pressure |
For pressure, see (25), (26). For NO see (27) |
gK1rel (algebraic relation: VSM membrane in proximal segment) |
total potassium channel conductivity in the proximal VSM segment |
The way that total potassium channel conductivity in the proximal VSM segment is the sum of the conductivities of the different types of channels |
|
gK2rel (algebraic relation: VSM membrane in distal segment) |
total potassium channel conductivity in the distal VSM segment |
The way that total potassium channel conductivity in the distal VSM segment is the sum of the conductivities of the different types of channels |
|
PD_mem1rel (algebraic relation: VSM membrane in proximal segment) |
membrane potential in proximal VSM segment |
How the membrane potential of the cells in the proximal VSM segment depends on the conductivity to and membrane potential for potassium, sodium and chlorine |
This is the linear model (p76 of (28)). Currently only the equilibrium potential for potassium and the potassium conductivity are treated as variables. Useful outlines of computational electrophysiology can be found here
and here. |
PD_mem2rel (algebraic relation: VSM membrane in distal segment) |
membrane potential in distal VSM segment |
How the membrane potential of the cells in the distal VSM segment depends on the conductivity to and membrane potential for potassium, sodium and chlorine |
See comments for PD_mem1rel |
gCa1rel (algebraic relation: VSM membrane in proximal segment) |
calcium channel conductivity in the proximal VSM segment |
How the conductivity of calcium channels in the proximal VSM segment depends on the membrane potential across the cells |
The functional form and parameter values are taken from (29) |
gCa2rel (algebraic relation: VSM membrane in distal segment) |
calcium channel conductivity in the distal VSM segment |
How the conductivity of calcium channels in the distal VSM segment depends on the membrane potential across the cells |
The functional form and parameter values are taken from (29) |
k_Cain1rel (algebraic relation: proximal VSM segment) |
rate of calcium entry into proximal VSM segment |
The equation determining the rate at which calcium enters VSM cells in the proximal arterial segment. |
This rate depends on the membrane potential, the membrane potential for calcium and the conductivity of the calcium channels |
k_Cain2rel (algebraic relation: distal VSM segment) |
rate of calcium entry into distal VSM segment |
The equation determining the rate at which calcium enters VSM cells in the distal arterial segment. |
This rate depends on the membrane potential, the membrane potential for calcium and the conductivity of the calcium channels |
NOprod1rel (algebraic relation: proximal VSM segment (plus implicitly, endothelium)) |
NO production rate in the proximal VSM segment |
The way that the rate of production of NO in the proximal VSM segment depends on the shear stress and extracellular pH |
|
NOprod2rel (algebraic relation: distal VSM segment (plus implicitly, endothelium)) |
NO production rate in the distal VSM segment |
The way that the rate of production of NO in the distal VSM segment depends on the shear stress and extracellular pH |
|
k_MLC1inacrel (algebraic relation: proximal VSM segment) |
inactivation rate of MLC in the proximal VSM segment |
The way in which the rate of inactivation (dephosphorylation) of myosin light chains in the proximal VSM segment depends on the concentration of NO |
The effect occurs via the activation of myosin light chain phosphatase - see (30) |
k_MLC2inacrel (algebraic relation: distal VSM segment) |
inactivation rate of MLC in the distal VSM segment |
The way in which the rate of inactivation (dephosphorylation) of myosin light chains in the distal VSM segment depends on the concentration of NO |
The effect occurs via the activation of myosin light chain phosphatase - see (30) |
k_MLC1acrel (algebraic relation: proximal VSM segment) |
activation rate of MLC in the proximal VSM segment |
The way in which the rate of activation (phosphorylation) of myosin light chains in the proximal VSM segment depends on the concentration of calcium |
The effect occurs via calcium binding to calmodulin and the complex subsequently activating myosin light chain kinase - see (16). Because of the complexity of the real pathway we simply choose a form which saturates for high concentrations of calcium and depends on some power of the concentration for small concentrations, and hope to be able to determine the parameters empirically. |
k_MLC2acrel (algebraic relation: distal VSM segment) |
activation rate of MLC in the distal VSM segment |
The way in which the rate of activation (phosphorylation) of myosin light chains in the distal VSM segment depends on the concentration of calcium |
see comments for k_MLC1acrel |
BBOrel (algebraic relation: capillary walls) |
diffusion rate for
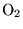 |
How the diffusion rate of
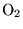 between blood and extracellular space is determined between blood and extracellular space is determined |
Currently the diffusion rate is set to be constant. But we allow for the possibility that it might depend on factors such as transmural pressure in future iterations of the model - see for example (31). Also on p15 of (32) it is stated that blood volume is an indicator of capillary surface area, though this remark isn't justified further. |
BBCrel (algebraic relation: capillary walls) |
diffusion rate for
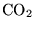 |
How the diffusion rate of
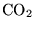 between blood and extracellular space is determined between blood and extracellular space is determined |
Currently this diffusion rate is set to be constant. But we allow for the possibility that it might depend on factors such as transmural pressure in future iterations of the model |
PD_Krel (algebraic relation: VSM membrane in both segments) |
membrane potential for potassium in VSM cells |
How the membrane potential for potassium in both VSM segments depends on the extracellular concentration of potassium |
Simply an application of the Nernst equation. See p52 of (28). |
PD_Ca1rel (algebraic relation: VSM membrane in proximal segment) |
membrane potential for calcium in the proximal VSM segment |
The way that PD_Ca1 - the membrane potential for calcium in the proximal VSM segment - depends on the intracellular concentration of calcium in the VSM cells |
Simply an application of the Nernst equation. See p52 of (28) |
PD_Ca2rel (algebraic relation: VSM membrane in distal segment) |
membrane potential for calcium in the distal VSM segment |
The way that PD_Ca2 - the membrane potential for calcium in the distal VSM segment - depends on the intracellular concentration of calcium in the VSM cells |
Simply an application of the Nernst equation. See p52 of (28) |
Vol_artrel (algebraic relation: ) |
Total arterial volume |
Equation determining the total arterial volume as the sum of the volumes of the proximal and distal arterial segments |
our add-on to the Ursino model in (1). |
Vol_venrel (algebraic relation: ) |
Total venous volume |
Equation determining the total venous volume |
our add-on to the Ursino model in (1). It is obtained by integrating the expressions for the two venous compartments, and assuming some baseline volume Vol_ven00 when the transmural pressure difference is zero |
| |
| table of processes ends |


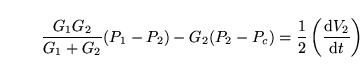
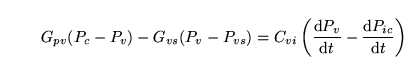
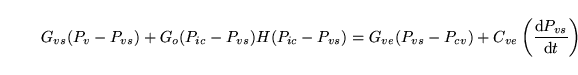
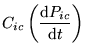
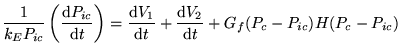
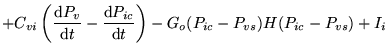
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() .
.
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() ,
,
![]() .
.